What is Threonine Metabolism?
Threonine, as an indispensable amino acid, occupies a central role in the intricate web of plant metabolism. Beyond merely participating in protein synthesis, it serves as a fundamental building block for essential bioactive compounds vital for plant health and development. Furthermore, its multifaceted role extends to acting as a signaling molecule, finely tuning the intricate processes of plant growth and development.
Within plant cells, threonine undergoes a complex metabolic journey, encompassing synthesis, degradation, and transformation into critical metabolites such as glycine and serine. This metabolic landscape holds significant implications across various domains, ranging from optimizing food processing techniques to bolstering agricultural yields and pushing the boundaries of pharmaceutical innovation. In this context, our threonine metabolism analysis service stands as a beacon of insight, offering a comprehensive understanding of threonine utilization pathways.
Threonine Metabolism Analysis Service by Creative Proteomics
Threonine Metabolism Metabolite Profiling: Leveraging an extensive library of reference standards and advanced analytical tools, Creative Proteomics offers precise identification and quantification of threonine and its metabolites in plant samples. This detailed profiling helps researchers understand threonine metabolic processes.
Threonine Metabolism Pathway Analysis: We also provide a comprehensive pathway analysis to map metabolites involved in threonine metabolism. Our service offers valuable insights into plant cell biochemistry, highlighting key regulatory points and metabolic fluxes.
Threonine Metabolism Enzyme Activity Assays: Specialized enzyme activity assays are used to measure the activity of key enzymes in threonine metabolism. These services explain metabolic flux changes and regulatory mechanisms by quantifying enzyme kinetics and substrate specificity.
Differential Metabolites Analysis In Threonine Metabolic Pathway: Creative Proteomics's differential metabolites analysis identifies and quantifies changes in metabolite levels under varying conditions. By comparing metabolic profiles, researchers can pinpoint differentially regulated metabolites.
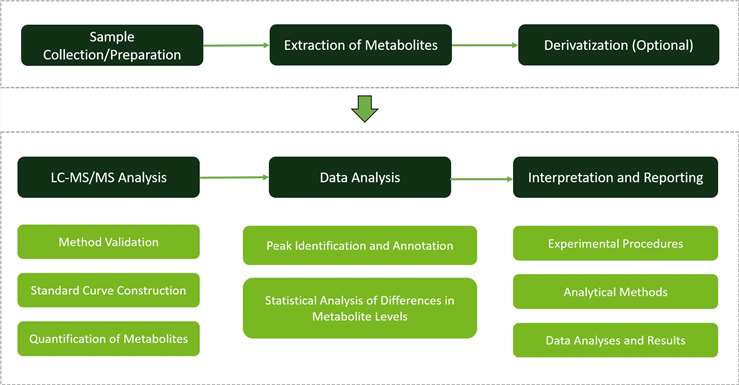
Techniques and Instrumentation for Threonine Metabolism Analysis
Liquid Chromatography (LC): At Creative Proteomics, liquid chromatography (LC) is a cornerstone technique utilized for the precise isolation and purification of threonine and its metabolites before subsequent mass spectrometry analysis. Our cutting-edge LC systems ensure exceptional chromatographic resolution, guaranteeing accurate quantification and identification of target compounds in complex biological samples.
Mass Spectrometry (MS): Mass spectrometry (MS) plays a pivotal role in the identification and quantification of threonine and its derivatives at Biotree. We leverage state-of-the-art MS platforms renowned for their high resolution and sensitivity. With these advanced tools, we achieve precise detection and quantification of threonine-related compounds across a diverse range of plant specimens, enabling comprehensive analysis of threonine metabolism.
LC-MS/MS Method Establishment and Optimization: Our approach involves establishing LC-MS/MS methods for targeted quantification of threonine and its metabolites. We construct standard curves using reference standards and employ multiple reaction monitoring (MRM) quantification to enhance detection efficiency and minimize interference, ensuring accurate and reproducible results across a wide concentration range.
Why Choose Us?
- Accurate Results: Our LC-MS/MS technique ensures precision through external standard curve quantification and internal standard calibration, guaranteeing reliability in threonine metabolism analysis. Each amino acid possesses an independent standard curve, facilitating precise measurement and discrimination of stereoisomers.
- Comprehensive Metabolite Coverage: Our analysis encompasses a diverse range of metabolites, providing extensive insights into threonine metabolism and its associated pathways, thus offering a comprehensive understanding of amino acid metabolism in plants.
- High-Throughput Analysis: With LC-MS/MS technology, we facilitate high-throughput analysis, enabling simultaneous quantification of up to 50 amino acids in a single run. This capability enhances analytical efficiency, expediting research progress and data acquisition.
- Key Point Detection: Our service excels in detecting critical points in the threonine metabolism pathway, offering broad coverage and comprehensive assessment of amino acid metabolic balance. This detailed analysis furnishes researchers with invaluable insights into threonine metabolism dynamics and regulation.
- Short Turnaround Time: We prioritize efficiency and promptness, delivering rapid service with short turnaround times. Our streamlined processes ensure timely data acquisition, enabling researchers to make informed decisions and progress their studies without delay.
Applications of Threonine Metabolism Analysis
Stress Response Studies: Threonine metabolism is integral to plant responses to stresses such as drought, salinity, and pathogen attacks. Our analysis identifies metabolic changes and adaptive mechanisms, aiding the development of stress-resistant crop varieties.
Nutrient Utilization Efficiency: Efficient nutrient use is crucial for sustainable agriculture. Profiling threonine metabolites provides insights into nitrogen assimilation and amino acid biosynthesis, leading to strategies that enhance nutrient use efficiency in plants.
Metabolic Engineering: For scientists in metabolic engineering, our service offers detailed insights into threonine biosynthesis and degradation pathways. This information is crucial for genetically engineering plants to produce desired metabolites or improve metabolic traits.
Functional Genomics: Linking gene function to metabolic outcomes is a key objective in plant genomics. Our threonine metabolism analysis supports functional genomics studies by correlating gene expression with metabolic profiles and elucidating gene function and regulation.
Sample Requirements for Threonine Metabolism Assay
| Plant Sample Type |
Minimum Quantity Required (fresh weight) |
Storage Conditions |
Additional Notes |
| Roots, Stems, Leaves, Fruits |
>200 mg |
Quick freeze in liquid nitrogen, Store at -80°C |
- |
| Seeds |
>200 mg |
Include seeds if applicable |
Each experimental treatment should have more than 6 biological replicates to ensure robust statistical analysis and reliable interpretation of results.
For other sample types not listed above, such as flowers or whole plants, please consult our technical support or sales team for specific requirements and recommendations.
Case . Hypothesis of Cell Wall Metabolism Disorder in Segment Drying: Evidence from Vesicle Collapse in 'Dayagan' Hybrid Citrus Fruit
Background:
Segment drying in citrus fruits, a physiological disorder occurring during ripening and postharvest storage, leads to nutrient loss and vesicle dysfunction.
This study investigates the "disorder of cell wall metabolism" hypothesis in vesicle collapse-type segment drying using 'Dayagan' hybrid citrus, aiming to understand the underlying metabolic changes and regulatory mechanisms.
Samples:
Mature 'Dayagan' hybrid citrus fruits were harvested from an orchard in January. Over 500 fruits of uniform size and shape were selected, with both normal and collapsed vesicles collected.
Samples were frozen in liquid nitrogen and stored at −80°C for further analysis.
Technical methods procedure:
Total soluble solids (TSS), titratable acidity (TA), relative water content (RWC), and juice yield were measured using standard methods. Each experiment had at least three replicates.
Widely targeted metabolomics and absolute quantification of amino acids were performed using UPLC-MS/MS. Differentially accumulated metabolites (DAMs) were identified with a VIP score ≥ 0.8 and P value < 0.05. Three biological replicates were used.
Cell wall components and calcium were extracted and quantified using modified standard methods. Experiments had a minimum of four replicates.
Activities of key enzymes (HXK, FrK, CS, GDH, GAD, ACO, GLN, ACL, PME, PG) were measured using commercial kits or established methods, with at least three replicates.
Genes related to carbohydrate, acid, and cell wall metabolism were identified in the Citrus sinensis genome. RNA-Seq was performed to analyze gene expression, identifying differentially expressed genes (DEGs) with an adjusted P value < 0.05.
Results
In 'Dayagan' citrus, segment drying begins in the stem region and is associated with a nutrient gradient.
Collapsed vesicles show significant changes: a 15% and 41% reduction in TSS and TA, respectively, and a decrease in juice yield (32% vs. 54%) without significant water loss.
Metabolomic analysis identified 944 metabolites, with 569 differentially accumulated. Key changes include a 52% decrease in sucrose, a 66% decrease in citrate, and increases in cell wall components like protopectin (32%) and cellulose (15%).
Enzyme activity assays revealed stable carbohydrate metabolism but increased activities for enzymes in the glutamine synthesis and acetyl-CoA pathways.
Transcriptomic analysis showed 6057 differentially expressed genes, with significant changes in pathways related to phenylpropanoid biosynthesis and glycolysis.
The findings suggest nutrient depletion and cell wall alterations are central to segment drying in 'Dayagan' fruit.
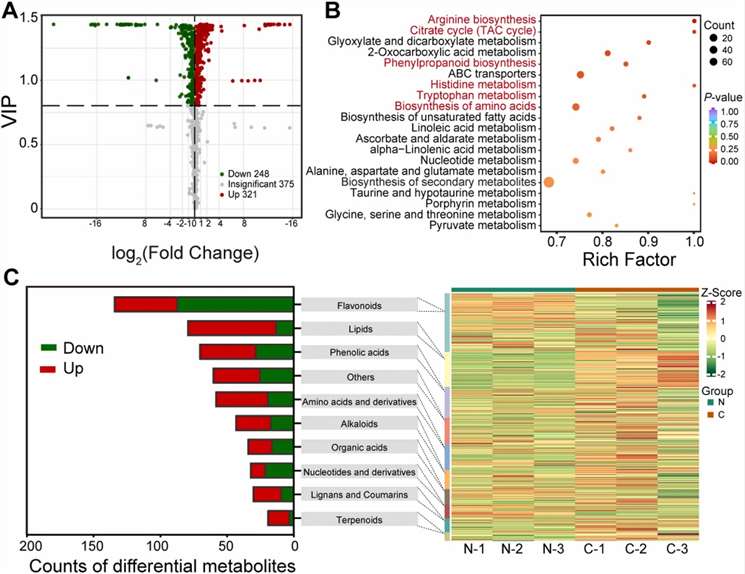 Fig 1. Widely targeted metabolomics profiling in pulp between normal and disordered fruit (affected by vesicle collapse). (A) Volcano map of differentially accumulated metabolites (DAMs); (B) scatter diagram of pathway enrichments of DAMs; (C) biochemical categories of DAMs and their hierarchical clustering.
Fig 1. Widely targeted metabolomics profiling in pulp between normal and disordered fruit (affected by vesicle collapse). (A) Volcano map of differentially accumulated metabolites (DAMs); (B) scatter diagram of pathway enrichments of DAMs; (C) biochemical categories of DAMs and their hierarchical clustering.
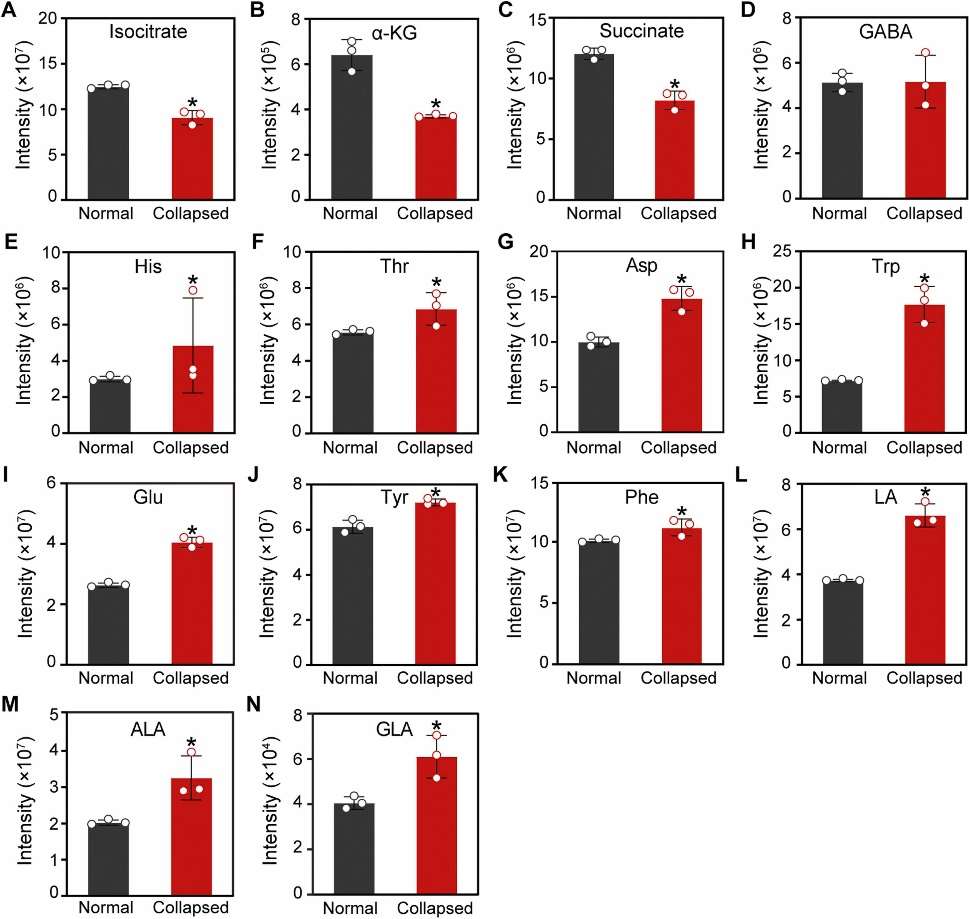 Fig 2. Contents of metabolites relating to citrate degradation of pulp between normal and disordered fruit (affected by vesicle collapse). (A) Isocitrate; (B) α-ketoglutaric acid (α-KG); (C) succinate; (D) γ-aminobutyric acid (GABA); (E) histidine (His); (F) threonine (Thr); (G) aspartate (Asp); (H) tryptophan (Trp); (I) glutamate (Glu); (J) tyrosine (Tyr); (K) phenylalanine (Phe); (L) linoleic acid (LA); (M) α-linolenic acid (ALA); (N) γ-linolenic acid (GLA). "Normal" and "Collapsed" means juice vesicles of the stem region from normal and segment-drying fruit, respectively.
Fig 2. Contents of metabolites relating to citrate degradation of pulp between normal and disordered fruit (affected by vesicle collapse). (A) Isocitrate; (B) α-ketoglutaric acid (α-KG); (C) succinate; (D) γ-aminobutyric acid (GABA); (E) histidine (His); (F) threonine (Thr); (G) aspartate (Asp); (H) tryptophan (Trp); (I) glutamate (Glu); (J) tyrosine (Tyr); (K) phenylalanine (Phe); (L) linoleic acid (LA); (M) α-linolenic acid (ALA); (N) γ-linolenic acid (GLA). "Normal" and "Collapsed" means juice vesicles of the stem region from normal and segment-drying fruit, respectively.
Reference
- Liu, Y. (2023). " Hypothesis of cell wall metabolism disorder in segment drying: Evidence from vesicle collapse in 'Dayagan' hybrid citrus fruit." Postharvest Biology and Technology 204, 112431.



 Fig 1. Widely targeted metabolomics profiling in pulp between normal and disordered fruit (affected by vesicle collapse). (A) Volcano map of differentially accumulated metabolites (DAMs); (B) scatter diagram of pathway enrichments of DAMs; (C) biochemical categories of DAMs and their hierarchical clustering.
Fig 1. Widely targeted metabolomics profiling in pulp between normal and disordered fruit (affected by vesicle collapse). (A) Volcano map of differentially accumulated metabolites (DAMs); (B) scatter diagram of pathway enrichments of DAMs; (C) biochemical categories of DAMs and their hierarchical clustering. Fig 2. Contents of metabolites relating to citrate degradation of pulp between normal and disordered fruit (affected by vesicle collapse). (A) Isocitrate; (B) α-ketoglutaric acid (α-KG); (C) succinate; (D) γ-aminobutyric acid (GABA); (E) histidine (His); (F) threonine (Thr); (G) aspartate (Asp); (H) tryptophan (Trp); (I) glutamate (Glu); (J) tyrosine (Tyr); (K) phenylalanine (Phe); (L) linoleic acid (LA); (M) α-linolenic acid (ALA); (N) γ-linolenic acid (GLA). "Normal" and "Collapsed" means juice vesicles of the stem region from normal and segment-drying fruit, respectively.
Fig 2. Contents of metabolites relating to citrate degradation of pulp between normal and disordered fruit (affected by vesicle collapse). (A) Isocitrate; (B) α-ketoglutaric acid (α-KG); (C) succinate; (D) γ-aminobutyric acid (GABA); (E) histidine (His); (F) threonine (Thr); (G) aspartate (Asp); (H) tryptophan (Trp); (I) glutamate (Glu); (J) tyrosine (Tyr); (K) phenylalanine (Phe); (L) linoleic acid (LA); (M) α-linolenic acid (ALA); (N) γ-linolenic acid (GLA). "Normal" and "Collapsed" means juice vesicles of the stem region from normal and segment-drying fruit, respectively.

