Flavanones are plant secondary metabolites that exhibit broad biological and pharmacological activities, including antioxidant, anti-inflammatory, and anticancer activities. These attributes have aroused tremendous interest in the pharmaceutical industry, driving the necessity for comprehensive flavanones analysis. Creative Proteomics, as a leading company with vast proficiency in the bio-analysis sector, specializes in conducting cutting-edge flavanones analysis, among other biological services.
What are Flavanones and Why Analyze Them?
Flavanones represent a sub-category of flavonoids, identifiable by their distinctive chemical structure, which typically contains a chromane-related ring and a substituted benzenoid. These compounds dominate in citrus fruits, where they are significantly associated with various health benefits like reducing inflammation, controlling diabetes, and other disease preventative attributes.
The necessity for analyzing flavanones revolves around their potential health benefits and their contribution to the overall pharmacokinetics of different plants and fruits. Flavanones analysis is an indispensable tool for assessing the quality and safety of dietary supplements containing citrus flavanones, recognizing new biomarkers, and contributing to food and pharmaceutical industrial growth.
Flavanones Analysis at Creative Proteomics
At Creative Proteomics, our specialized flavonoid analysis services meet a variety of research needs, from understanding their structural identification to characterizing their metabolic profile and scrutinizing their biological activity.
- Structural Identification: Leveraging state-of-the-art technologies such as mass spectrometry and nuclear magnetic resonance (NMR), we proficiently identify and quantify the flavanone compounds from various samples, be it plant sources or biological fluids.
- Metabolic Profile Characterization: With our advanced LC-MS/MS and GC-MS technologies, we can study the metabolic profiles of flavanones in specific biological systems, identifying the metabolites involved and the pathways they undergo.
- Biological Activity Determination: Our capable team at Creative Proteomics also evaluates the potential biological activities of flavanones, utilizing cutting-edge equipment to examine their interaction with proteins, nucleic acids, and other cell components.
It's essential to mention that our flavanones analysis service adheres to the highest industry standards, guaranteeing credible and reliable results for academics and industrial research alike.
Flavanones Analysis Techniques
High-Performance Liquid Chromatography (HPLC): HPLC coupled to UV detection is one of the most common techniques used for the separation and quantification of flavanones. For instance, Agilent 1260 Infinity II or Waters Acquity HPLC system can be used.
Mass Spectrometry (MS): MS is often used in conjunction with HPLC for the identification of flavanones. This technique allows for the separation of ions based on their mass-to-charge ratios. Examples of MS instruments include the Thermo Fisher Scientific Orbitrap Fusion Series and the Agilent 6546 LC/Q-TOF.
Gas Chromatography-Mass Spectrometry (GC-MS): GC-MS is an analytical method that combines the features of gas chromatography and mass spectrometry to identify different substances within a test sample. An example of a GC-MS instrument is the Agilent 7890B GC system combined with an Agilent 5977A Series GC/MSD System.
Nuclear Magnetic Resonance (NMR) Spectroscopy: NMR can also be used for the structural elucidation of flavanones. Instruments for NMR include the Bruker Avance III HD.
Liquid Chromatography-Mass Spectrometry (LC-MS): LC-MS is a technique often used in drug discovery and food safety analysis. For example, the Thermo Scientific™ Q Exactive™ Hybrid Quadrupole-Orbitrap™ mass spectrometer can be used for this analysis.
 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service
List of Flavanones Analyzed (including but not limited to)
| Astilbin |
Eriocitrin |
Eriodictyol |
Hesperetin |
Hesperetin 7-rhamnoglucoside |
| Hesperidin |
Hesperidin methyl chalcone |
Isosakuranetin |
Naringenin |
Naringin |
| Naringin dihydrochalcone |
Narirutin |
Neohesperidin |
Pinocembrin |
Poncirin |
| Homoeriodictyol |
|
|
|
|
Sample Requirements for Flavanones Assay
| Sample Type |
Sample Volume |
Additional Notes |
| Plant Extracts |
1 mL |
Use organic solvents for extraction. |
| Biological Fluids |
500 µL |
Precipitate proteins before analysis. |
| Food Products |
2 g |
Homogenize samples for uniformity. |
| Cosmetic Formulations |
1 mL |
Consider extraction efficiency for analysis. |
| Environmental Samples |
5 mL |
Filter samples to remove particulate matter. |
| Herbal Supplements |
1 g |
Ensure proper extraction for complex matrices. |
| Pharmaceutical Formulations |
1 mL |
Check for potential interference from excipients. |
| Tissue Homogenates |
100 mg |
Optimize homogenization for effective extraction. |
| Blood Plasma |
200 µL |
Anticoagulants may be required for plasma samples. |
| Soil Samples |
5 g |
Extract flavanones using suitable solvents. |
Deliverables of Flavanones Analysis
- A meticulous consult and a well set out plan towards your project requirements.
- Extensive analysis of the flavanones present in the samples.
- A comprehensive report that includes experimental procedures, parameters, and results interpretation.
- Queried database files.
- Stringent quality control routines ensuring the accuracy and reliability of the data.
Case. Probiotic Impact on Flavanone Metabolism
Background:
This study explores the impact of probiotic strains, Lactobacillus rhamnosus and Bifidobacterium longum, on the catabolism of flavanones. Understanding how these probiotics interact with flavanones can provide insights into their potential health effects, especially in the context of bioavailability and metabolic transformations.
Sample:
The research utilized strains of Lactobacillus rhamnosus subsp. Rhamnosus NCTC 10302 and Bifidobacterium longum R0175. Flavanones, including naringenin and hesperetin, along with their 7-O-rutinosides (hesperidin and narirutin), were employed as the target compounds.
Technical Platform and Procedure:
Chemicals and Reagents: Flavanones and standards were procured from reputable suppliers. Enzymes, buffers, and HPLC–MS-grade methanol were used from reliable sources.
Bacterial Strains and Growth Conditions: L. rhamnosus and B. longum were cultured in specific media under anaerobic conditions.
Effect of Flavanones on Bacterial Growth: The impact of flavanones on probiotic growth was assessed by monitoring OD620 over incubation periods.
Enzyme Activity Assay: β-Glucosidase and α-rhamnosidase activities were measured using specific substrates, and enzyme activity was quantified.
Incubation of Flavanones with Probiotics: The catabolism of flavanones by L. rhamnosus and B. longum was studied through incubations, and samples were collected at different time points.
Identification and Quantification: HPLC–HR–MS analysis was conducted for the identification and quantification of flavanones and their catabolites.
Results
Effect on Bacterial Growth: L. rhamnosus tolerated flavanones up to 50 µg/mL, while B. longum exhibited growth inhibition at concentrations ≥50 µg/mL.
Enzymatic Activity: L. rhamnosus showed higher α-rhamnosidase activity, and both strains exhibited similar β-glucosidase activity.
Bacterial Degradation of Flavanones: Both strains catabolized flavanones, producing specific catabolites.
Identification and Quantification: Catabolites included 3-(3′-hydroxy-4′-methoxyphenyl)propionic acid, 3-(3′,4′-dihydroxyphenyl)propionic acid, and others.
Overall Recovery and Bioavailability: The recovered catabolites represented the full spectrum of flavanone-derived compounds. Chronic intake of B. longum R0175 enhanced flavanone bioavailability.
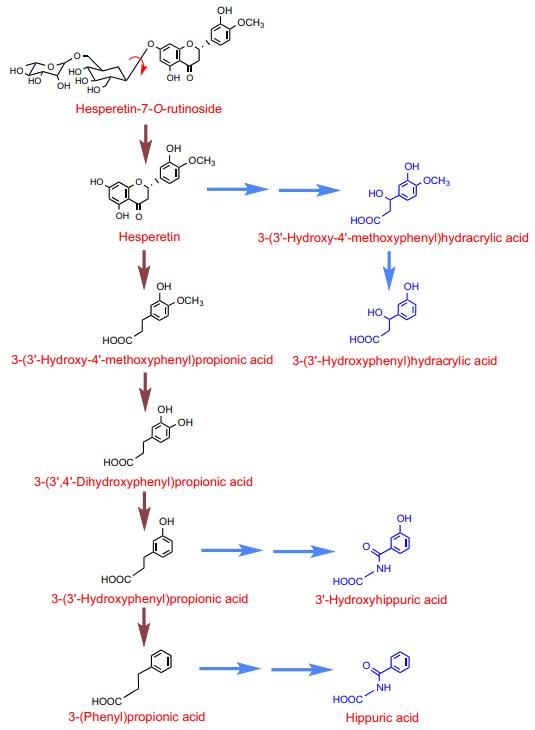 Proposed pathway for the catabolism of hesperetin-7-O-rutinoside.
Proposed pathway for the catabolism of hesperetin-7-O-rutinoside.
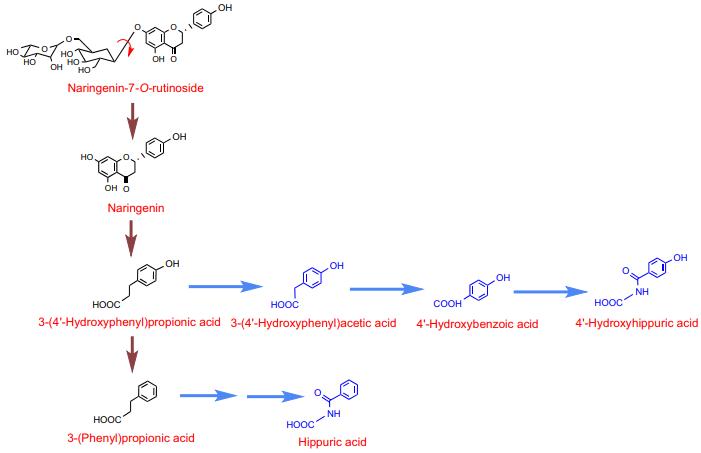 Proposed pathway for the catabolism of naringenin-7-Orutinoside
Proposed pathway for the catabolism of naringenin-7-Orutinoside
Reference
- Pereira-Caro, Gema, et al. "Catabolism of citrus flavanones by the probiotics Bifidobacterium longum and Lactobacillus rhamnosus." European journal of nutrition 57 (2018): 231-242.


 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service Proposed pathway for the catabolism of hesperetin-7-O-rutinoside.
Proposed pathway for the catabolism of hesperetin-7-O-rutinoside. Proposed pathway for the catabolism of naringenin-7-Orutinoside
Proposed pathway for the catabolism of naringenin-7-Orutinoside

