What is Kaempferol?
Kaempferol is a flavonoid, a class of naturally occurring compounds characterized by their diverse biological activities and widespread presence in the plant kingdom. Specifically, kaempferol belongs to the subgroup of flavonols, which are known for their antioxidant properties. It is a polyphenolic compound, meaning it contains multiple phenol functional groups in its chemical structure. The chemical structure of kaempferol consists of three rings: two benzene rings (A and B rings) and a pyrone ring (C ring) with a ketone group. The specific arrangement of these rings and functional groups contributes to kaempferol's unique properties and biological activities.
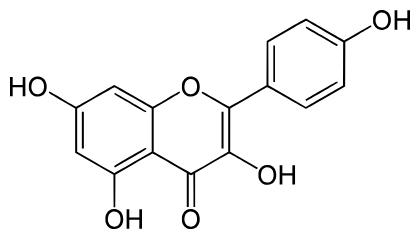 Kaempferol
Kaempferol
Kaempferol, potential in anti-oxidational, anti-inflammational, and anti-cancerous activities substantiated by numerous studies, begs the magnifying lens of science for a detailed understanding of its functionalities at a molecular level. Consequently, it also provides a pathway for developing novel medicinal preparations and augmenting the nutritional content of foods.
Moreover, it is critical to determine the accurate quantities and purity of kaempferol and its derivatives in different plant sources for quality control purposes. An in-depth analysis can also identify the presence of any related impurities or adulterants, ensuring the safety and efficacy of the end product.
Additionally, a thorough understanding of the bioavailability, metabolism, and kinetic properties of kaempferol in humans requires its detailed analysis.
At Creative Proteomics, our services go above and beyond simple qualitative and quantitative analysis, offering comprehensive assessments of kaempferol's chemical structure, sources, biological activity, and metabolism.
Specific Kaempferol Analysis Offered by Creative Proteomics
At Creative Proteomics, we have developed a reliable and versatile kaempferol analysis platform that can cater to various scientific research and industrial needs. Our platform focuses on the following project directions:
- Quantitative Analysis: Accurate measurement of kaempferol levels in different samples, providing quantitative data for research and quality control purposes.
- Qualitative Analysis: Identification and characterization of kaempferol in complex sample matrices, ensuring the purity and authenticity of the compound.
- Metabolite Profiling: Profiling and identification of kaempferol metabolites in biological samples to understand its metabolic fate and potential bioactivity.
- Bioavailability Studies: Investigation of kaempferol's bioavailability in different formulations or delivery systems, providing insights for optimizing its therapeutic or nutritional effects.
- Structural Elucidation: Detailed structural analysis of kaempferol and its derivatives, enabling a deeper understanding of its chemical composition and properties.
- Stability Studies: Evaluation of kaempferol stability under various conditions, aiding in the development of stable formulations for pharmaceutical or nutraceutical applications.
- Toxicity Assessment: Assessment of the potential toxicity of kaempferol to ensure its safety for use in different applications.
- Biosynthesis Pathway Analysis: Investigation of the biosynthetic pathways involved in kaempferol production, contributing to the understanding of its natural synthesis in plants.
- Customized Analytical Solutions: Tailored kaempferol analysis solutions based on specific client needs and research objectives.
Our professional and experienced team of experts, armed with advanced analytical instruments and backed by our proven track record in the field of biosciences, ensures our clients receive robust, accurate, and comprehensive kaempferol analysis results.
Kaempferol Analysis Techniques
Liquid Chromatography-Mass Spectrometry (LC-MS): This technique excellently separates, identifies, and quantifies kaempferol even in complex samples.
Gas Chromatography-Mass Spectrometry (GC-MS): Acknowledged for separate volatile compounds and help in the deducing the structure of kaempferol.
Matrix-Assisted Laser Desorption/Ionization Time of Flight Mass Spectrometry (MALDI-TOF MS): A powerful analytical tool that helps in the detection and identification of kaempferol and its derivatives.
 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service
List of Kaempferol and Its Derivatives Analyzed (including but not limited to)
| Kaempferol |
Kaempferide |
Kaempferol-3-glucoside |
Kaempferol-3-rutinoside |
| Kaempferol-7-glucoside |
Kaempferol-4'-glucoside |
|
|
Sample Requirements for Kaempferol Assay
| Sample Type |
Recommended Quantity |
| Plant Extracts |
10-50 mg |
| Dietary Sources |
5-20 g |
| Herbal Supplements |
1-5 g |
| Tea Leaves |
2-10 g |
| Cell Cultures |
1-5 million cells |
| Serum/Plasma |
0.5-2 mL |
| Urine |
5-20 mL |
| Tissues (Biopsy) |
10-50 mg |
Deliverables from Kaempferol Analysis
- A detailed report with all the methodologies and techniques used, and the results are incorporated in a comprehensive and easy-to-understand manner.
- Raw data files, processed data files and supporting documentation if required.
- Recommendations and insights drawn from the data analysis.
Case. Development and Application of LC–MS/MS Method for Pharmacokinetic Study of Flavonols in Verbena officinalis L. Extract in Rat Plasma.
Background
Traditional Chinese Medicine (TCM) plays a significant role in healthcare, and the pharmacokinetic study of medicinal herbs like Verbena officinalis L. is crucial for understanding their therapeutic effects. This study aims to develop a sensitive LC–MS/MS method for analyzing flavonols in rat plasma after the oral administration of Verbena officinalis L. extract.
Sample
Rat plasma samples were utilized for the pharmacokinetic study. Two sets of samples were prepared: set 1 contained reference standards dissolved in water and methanol–hydrochloric acid, while set 2 included blank plasma samples spiked with the same concentrations of analytes.
Technical Platform and Procedure
Mass Spectra Analysis: Flavonols such as luteolin, kaempferol, apigenin, quercetol, and isorhamnetin were characterized using mass spectra obtained through syringe pump infusion analysis. Negative ion mode was chosen for enhanced sensitivity.
Liquid Chromatographic Conditions: The mobile phase comprised acetonitrile–0.01% formic acid, offering improved sensitivity and lower background noise. Matrix effect evaluation indicated the optimal conditions for efficient electrospray ionization.
Sample Preparation: Liquid–liquid extraction technique was deemed unsuitable, leading to a simple protein precipitation procedure using methanol–25% hydrochloric acid. Acid hydrolysis was incorporated for the determination of total flavone concentration.
Selection of Internal Standard (IS): Sulfamethazine (SMZ) was chosen as the internal standard due to its compatibility with flavones, providing clean chromatography with minimal interference.
Results
Specificity: Chromatograms demonstrated excellent resolution and peak shapes with no interfering peaks, confirming the high selectivity of the Multiple Reaction Monitoring (MRM) mode.
Linearity: Regression equations showed excellent correlation between peak area and concentration, with low limits of detection (LODs) and quantification (LOQs).
Precision, Accuracy, and Extraction Recovery: The method exhibited high precision and accuracy in intra- and inter-day analyses, with extraction recoveries within acceptable ranges.
Stability: Stability data indicated that the analytes in plasma remained stable for 24 hours in autosampler conditions and after at least three freeze-thaw cycles.
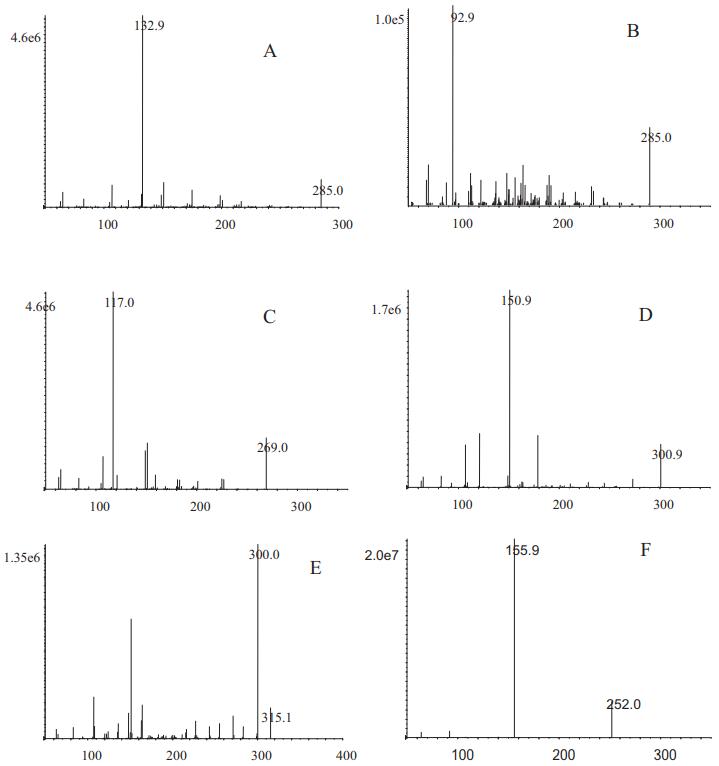 MS and product ion spectra of the compounds. Luteolin (A), kaempferol (B), apigenin (C), quercetol (D), isorhamnetin (E) and IS (F).
MS and product ion spectra of the compounds. Luteolin (A), kaempferol (B), apigenin (C), quercetol (D), isorhamnetin (E) and IS (F).
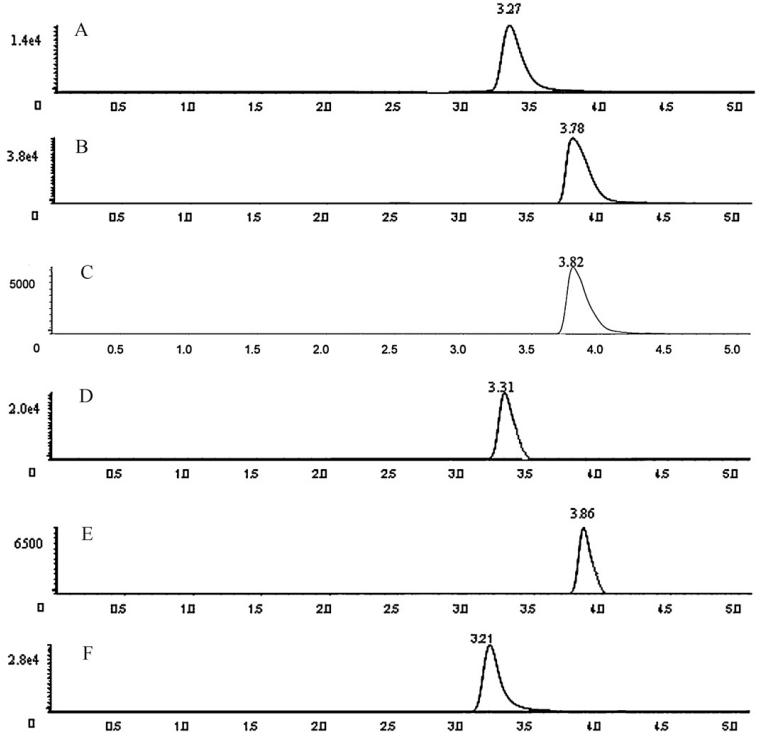 Typical MRM chromatograms of analytes (A–E) and IS (F) in rat plasma sample colleted at 30 min after oral administration of Verbena officinalis L. extract. Luteolin (A), kaempferol (B), apigenin (C), quercetol (D), isorhamnetin (E) and IS (F).
Typical MRM chromatograms of analytes (A–E) and IS (F) in rat plasma sample colleted at 30 min after oral administration of Verbena officinalis L. extract. Luteolin (A), kaempferol (B), apigenin (C), quercetol (D), isorhamnetin (E) and IS (F).
Reference
- Duan, Kunfeng, et al. "LC–MS/MS determination and pharmacokinetic study of five flavone components after solvent extraction/acid hydrolysis in rat plasma after oral administration of Verbena officinalis L. extract." Journal of ethnopharmacology 135.2 (2011): 201-208.


 Kaempferol
Kaempferol Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service MS and product ion spectra of the compounds. Luteolin (A), kaempferol (B), apigenin (C), quercetol (D), isorhamnetin (E) and IS (F).
MS and product ion spectra of the compounds. Luteolin (A), kaempferol (B), apigenin (C), quercetol (D), isorhamnetin (E) and IS (F). Typical MRM chromatograms of analytes (A–E) and IS (F) in rat plasma sample colleted at 30 min after oral administration of Verbena officinalis L. extract. Luteolin (A), kaempferol (B), apigenin (C), quercetol (D), isorhamnetin (E) and IS (F).
Typical MRM chromatograms of analytes (A–E) and IS (F) in rat plasma sample colleted at 30 min after oral administration of Verbena officinalis L. extract. Luteolin (A), kaempferol (B), apigenin (C), quercetol (D), isorhamnetin (E) and IS (F).

