What is Diterpene?
Diterpenes represent a diverse class of terpenes characterized by a molecular framework derived from four isoprene units, typically corresponding to the molecular formula C20H32. These compounds, synthesized through the HMG-CoA reductase pathway in various organisms including plants, fungi, and some bacteria, are precursors to numerous biologically significant molecules, such as phytol, taxadiene, and retinoids. The structural diversity of diterpenes is a product of complex biosynthetic pathways involving cyclization and chemical modifications, rendering them crucial in pharmacology, agriculture, and biotechnological applications. The precise analysis of diterpenes is, therefore, vital for various research and industrial purposes.
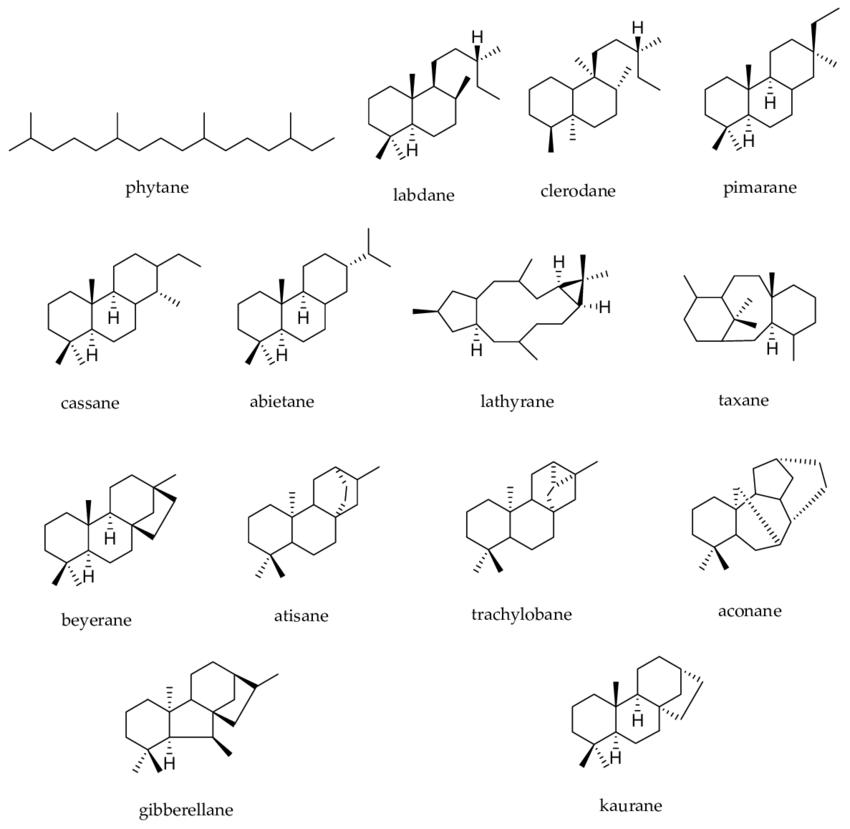
Diterpene analysis is crucial for understanding the chemical composition and biological activity of diterpenes, which are key in pharmaceuticals, flavors, and fragrances. It ensures the quality and safety of diterpene-containing products by identifying and quantifying these compounds, aiding in quality control and the development of new therapeutic agents.
Techniques and Instrumentation for Diterpene Analysis
Creative Proteomics employs cutting-edge techniques and instrumentation to ensure the highest accuracy and sensitivity in diterpene analysis. Our analytical platforms include:
Liquid Chromatography-Mass Spectrometry (LC-MS)
LC-MS, particularly High-performance liquid chromatography (HPLC) coupled with high-resolution MS (HRMS), is the cornerstone of our diterpene analysis. This technique allows for the separation, identification, and quantification of diterpenes with high precision.
Gas Chromatography-Mass Spectrometry (GC-MS)
GC-MS is employed for the analysis of volatile diterpenes and those amenable to gas-phase analysis. Our systems include collision-induced dissociation (CID) and higher-energy collisional dissociation (HCD) sources, enabling the detection of diterpenes with varying volatilities and polarities.
Types of Diterpenes Analytical Services
Creative Proteomics offers a comprehensive suite of Diterpene Analysis Services tailored to meet the diverse needs of our clients in various industries, including pharmaceuticals, agriculture, and cosmetics. As an integral part of our plant target metabolomics suite, these services are tailored to provide precise and reliable data for a wide range of diterpenes.
Qualitative and Quantitative Analysis for Diterpenes
Identification of Diterpenes: Utilizing advanced MS techniques, accurately identifying the structure of diterpenes present in complex mixtures.
Quantification of Diterpenes: HPLC and GC coupled with MS are employed for the precise quantification of diterpenes in various matrices.
Diterpenes Structural Elucidation
Cyclization Pattern Analysis: Determination of cyclization patterns through detailed MS analysis, enabling the identification of the carbon skeleton and functional groups.
Isomer Differentiation: Through chiral chromatography and MS, we differentiate between isomeric forms of diterpenes, which is essential for understanding their distinct biological activities.
Diterpenes Biosynthetic Pathway Analysis
Enzyme Activity Assays: We offer assays to study the activity of diterpene synthases and other enzymes involved in diterpene biosynthesis, providing insights into the metabolic pathways.
Stability and Degradation Studies
Degradation Pathway Elucidation: We identify and quantify degradation products formed under various conditions, utilizing LC-MS and GC-MS.
List of Diterpenes Analyzed (including but not limited to)
| Taxadiene |
Taxanes |
Gibberellins |
Phytol |
Retinoids |
| Forskolin |
Abietic Acid |
Forskolin |
Triptonide |
Cafestol |
| Kahweol |
Isocupressic Acid |
Labdane |
Marrubiin |
Oridonin |
| Aconitine |
Sclareol |
Cembrene |
Cycloartenol |
Milbemycin |
Service Components for Diterpenes Analysis
Gas Chromatography-Mass Spectrometry (GC-MS)
GC-MS is a powerful tool for the separation and identification of volatile sesquiterpenes. The technique involves the vaporization of the sample, followed by the separation of its components in a gas chromatograph. The separated compounds are then ionized and detected by mass spectrometry, which provides both qualitative and quantitative data. GC-MS is particularly effective for analyzing hydrocarbon sesquiterpenes and their oxygenated derivatives, allowing for detailed profiling of complex mixtures.
Liquid Chromatography-Mass Spectrometry (LC-MS)
For non-volatile or thermally labile sesquiterpenes, LC-MS is the preferred method. This technique involves the separation of sesquiterpenes in a liquid chromatograph, followed by detection via mass spectrometry. LC-MS offers high sensitivity and specificity, making it ideal for the analysis of sesquiterpene lactones, alcohols, acids, and other derivatives. The combination of LC with tandem mass spectrometry (LC-MS/MS) allows for structural elucidation and accurate quantification, even in complex biological matrices.
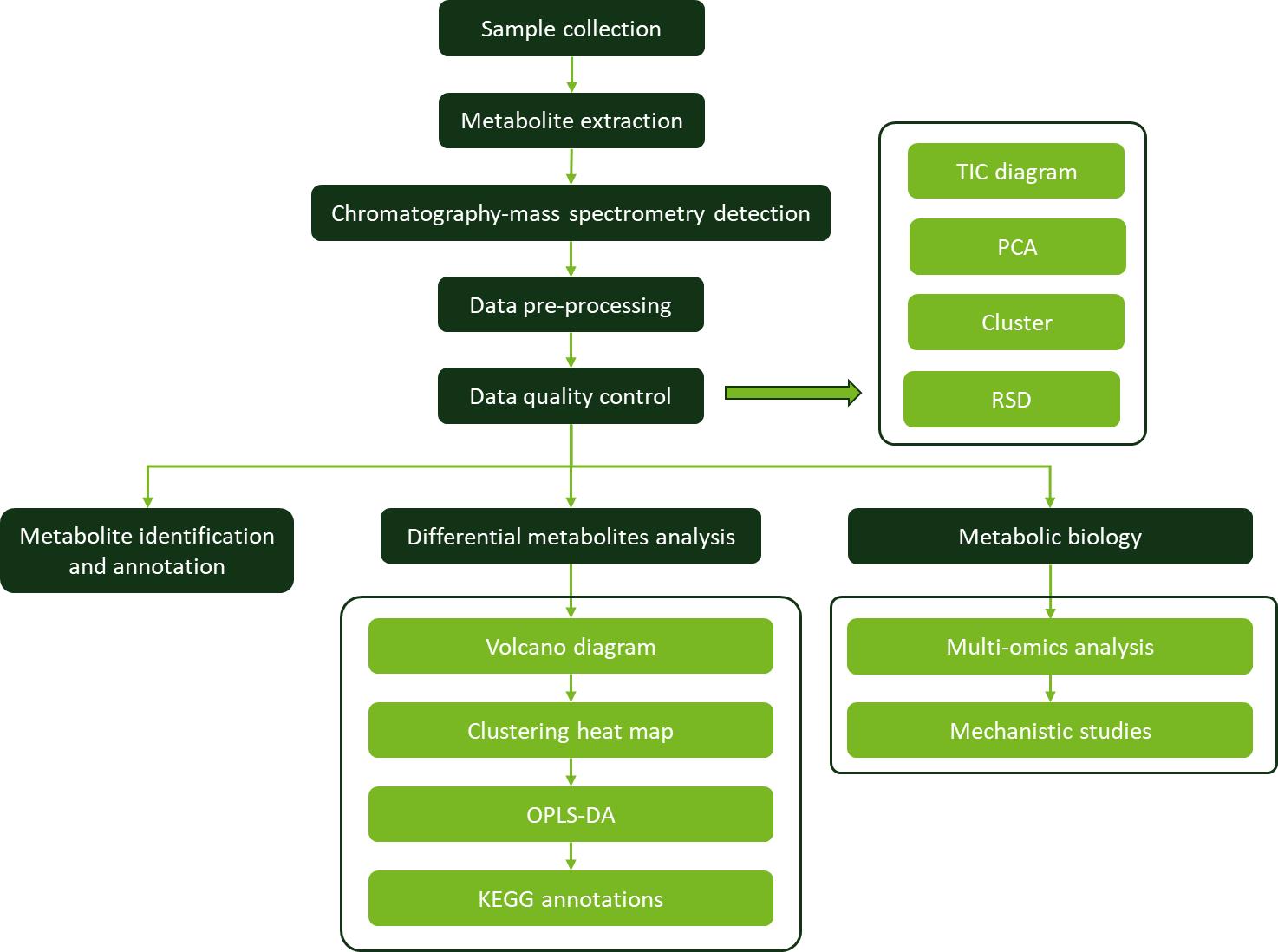
Why Need Diterpenes Analysis?
The comprehensive analysis of diterpenes has wide-ranging applications across various industries:
Pharmaceutical Development: Diterpenes analysis are integral to the development of drugs, especially in oncology and anti-inflammatory therapies.
Agricultural Biotechnology: Diterpenes play a significant role in plant defense mechanisms and are utilized in developing bio-pesticides and growth regulators.
Environmental Studies: Analysis the presence of diterpenes in environmental samples, such as soil and water, can indicate specific biological activities or contamination events.
Food and Flavor Industry: Diterpenes analysis contribute to the aroma and flavor profiles of various foods.
Synthetic Biology: The ability to profile and analyze diterpenes enables the optimization of biosynthetic pathways in microorganisms, leading to the sustainable production of valuable diterpene compounds.
Sample Requirements for Diterpenes Analysis
| Sample Types |
Requirements |
| Plant Samples |
Dried or fresh plant tissues, including leaves, stems, roots, or whole plant extracts. |
- Minimum weight: 500 mg dry weight or 5 g fresh weight
- Properly labeled and packaged |
| Liquid Samples |
Extracts from plant tissues, culture media, or other biological fluids. |
- Minimum volume: 5 mL
- Properly sealed in sterile containers |
| Specialty Samples |
-Purified diterpenes, fractionated samples, or chemically modified derivatives.
-environmental or food samples for specific assays. |
- Custom requirements based on sample type (e.g., purified compounds, extracts) |
Case. Direct and comprehensive analysis of ginsenosides and diterpene alkaloids in Shenfu injection by combinatory liquid chromatography–mass spectrometric techniques
Background
Shenfu Injection (SFI) is a traditional Chinese herbal formulation used for treating cardiovascular diseases. It is derived from red ginseng (Panax ginseng) and processed aconite root (Aconitum carmichaeli). Despite its extensive clinical use and established pharmacological effects, there is limited research on its chemical composition and quality control, particularly regarding toxic aconite alkaloids. To address this, the study applied high-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry (HPLC–QTOF MS) to analyze the constituents of SFI comprehensively.
Samples
The study analyzed Shenfu Injection samples from five different batches (Batch No. 110108, 110805, 120108, 120703, 111011) obtained from Ya'an Sanjiu Pharmaceutical Co., Ltd.
Reference compounds for analysis included various alkaloids and ginsenosides purchased or isolated in the authors' laboratory.
Technical Methods Procedure
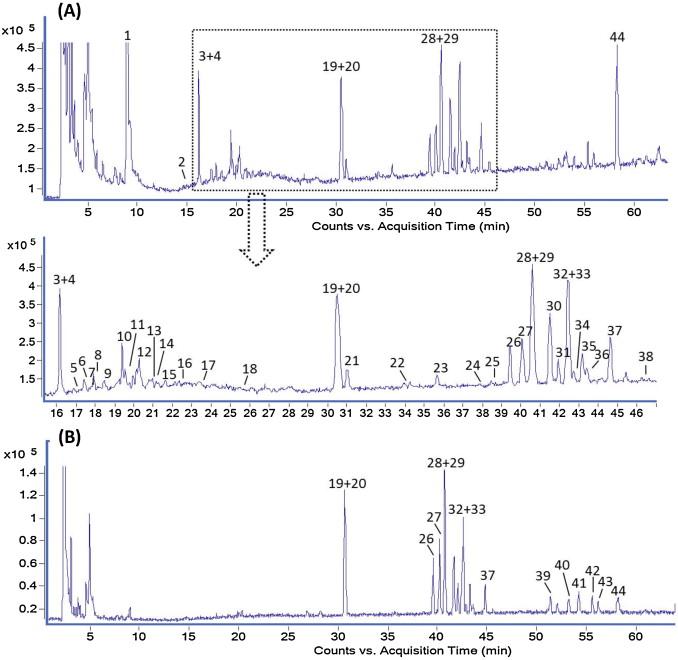
Qualitative Analysis: HPLC–QTOF MS was used to profile the components in SFI. Samples were analyzed in positive ion mode for alkaloids and negative ion mode for ginsenosides. Chromatographic separation was achieved using an Agilent Zorbax SB-C18 column with a specific gradient elution program. The QTOF MS parameters included a range of 100 to 3000 m/z and settings for both positive and negative ion modes.
Quantitative Analysis: An LC-MS method with selected ion monitoring (SIM) quantified 20 major alkaloids and ginsenosides. The method was validated for linearity, accuracy, and precision, with low limits of quantification (LOQ) ranging from 0.4 ng/mL to 18 ng/mL for diterpene alkaloids.
Results
Qualitative Findings: A total of 44 compounds were identified in SFI, including 23 diterpene alkaloids, 19 ginseng saponins, one panaxytriol, and one 5-hydroxymethylfurfural.
Quantitative Findings: The LC-MS method successfully quantified the major alkaloids and ginsenosides in the SFI samples. The total concentrations of saponins and alkaloids were measured to be about 676-742 μg/mL and 3-7 μg/mL, respectively. The batch-to-batch reproducibility was evaluated using cosine ratio and euclidean distance, demonstrating high consistency in product quality.
Reference
- Yang, Hua, et al. "Direct and comprehensive analysis of ginsenosides and diterpene alkaloids in Shenfu injection by combinatory liquid chromatography–mass spectrometric techniques." Journal of Pharmaceutical and Biomedical Analysis 92 (2014): 13-21.











