What is Eriodictyol?
Eriodictyol, chemically known as 3',4',5,7-tetrahydroxyflavanone, is a flavanone compound commonly found in various plant sources, including citrus fruits, parsley, and cocoa. Its chemical structure comprises a flavanone backbone with hydroxyl groups at specific positions, imparting antioxidant, anti-inflammatory, and anticancer properties. Studies have shown that eriodictyol exhibits a range of pharmacological effects, such as scavenging free radicals, modulating cellular signaling pathways, and inhibiting the growth of tumor cells.
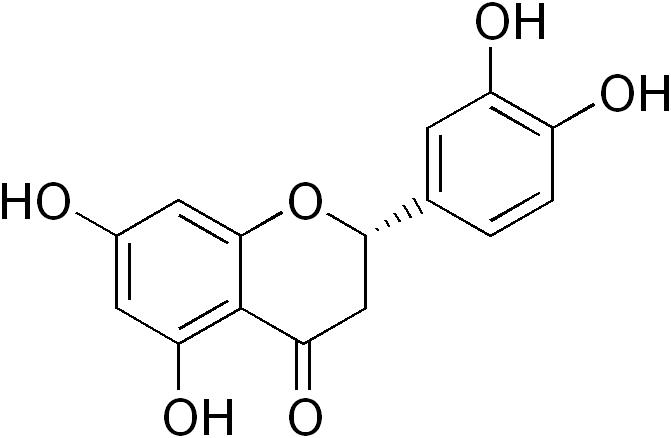 Eriodictyol
Eriodictyol
The increasing interest in eriodictyol stems from its potential therapeutic applications in various health conditions, including cardiovascular diseases, neurodegenerative disorders, and cancer. However, the efficacy and safety of eriodictyol-containing products depend significantly on the compound's concentration and purity. Therefore, accurate analysis of eriodictyol content is crucial for quality control, pharmacokinetic studies, and regulatory compliance in industries such as pharmaceuticals, nutraceuticals, and food supplements.
Creative Proteomics offers a comprehensive suite of eriodictyol analysis services tailored to meet the diverse needs of researchers and industries.
Eriodictyol Analysis Offered by Creative Proteomics
Eriodictyol Quantitative Analysis
Our quantitative eriodictyol analysis service allows precise determination of eriodictyol concentration in various sample matrices, including plant extracts, dietary supplements, and biological fluids. Leveraging advanced liquid chromatography-mass spectrometry (LC-MS) techniques, we provide quantitative data with exceptional sensitivity and specificity, enabling reliable assessment of eriodictyol levels for research and product development purposes.
Eriodictyol Qualitative Analysis
In addition to quantitative analysis, Creative Proteomics offers qualitative eriodictyol analysis to identify and characterize eriodictyol-containing compounds in complex mixtures. Through high-resolution mass spectrometry coupled with tandem mass spectrometry (MS/MS), we elucidate the molecular structure and fragmentation patterns of eriodictyol and its derivatives, facilitating comprehensive metabolomic profiling and compound identification studies.
Eriodictyol Stability and Degradation Studies
Understanding the stability and degradation kinetics of eriodictyol is essential for optimizing formulation strategies and ensuring product quality over time. Creative Proteomics conducts stability testing and degradation studies to evaluate the effects of various environmental factors, such as temperature, light, and pH, on the integrity and shelf-life of eriodictyol-containing formulations.
Technological Platforms for Eriodictyol Analysis
At Creative Proteomics, our eriodictyol analysis service is built around advanced mass spectrometry (MS) platforms. These platforms allow for the precise quantification and detailed characterization of eriodictyol in a wide range of samples. We utilize the following high-end instruments for our analysis:
Liquid Chromatography-Mass Spectrometry (LC-MS/MS): Our state-of-the-art LC-MS/MS systems, including the Thermo Fisher Scientific™ Q Exactive™ HF Hybrid Quadrupole-Orbitrap™ and the Agilent 6550 iFunnel Q-TOF, are at the forefront of our eriodictyol analysis service. These instruments offer unmatched sensitivity, resolution, and accuracy, enabling the detection of eriodictyol at very low concentrations.
Ultra-High-Performance Liquid Chromatography (UHPLC): Coupled with MS, our UHPLC systems, such as the Waters™ ACQUITY UPLC™, enhance the separation efficiency and reduce analysis time, significantly improving the throughput and reliability of eriodictyol detection.
 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service
Sample Requirements for Eriodictyol Assay
| Sample Type |
Sample Volume |
Sample Preparation |
| Plant Extracts |
100-500 µL |
- Dilute with appropriate solvent (e.g., methanol, ethanol) |
| Biological Fluids |
100-500 µL |
- Extract using appropriate method (e.g., liquid-liquid extraction) |
| Herbal Formulations |
50-200 mg |
- Extract using suitable solvent and method |
| Cell Culture Supernatant |
100-500 µL |
- Centrifuge to remove debris, then analyze supernatant |
| Tissue Samples |
10-50 mg |
- Homogenize in appropriate buffer or solvent |
| Food and Beverage |
5-20 mL |
- Homogenize or extract using appropriate solvent and method |
| Dietary Supplements |
50-200 mg |
- Dissolve in suitable solvent (e.g., methanol, ethanol) |
Deliverables of Eriodictyol Analysis
Quantitative and Qualitative Analysis Reports: Detailed reports providing quantitative measurements of eriodictyol in your samples, along with qualitative insights into its molecular structure and properties.
Methodology Documentation: Full documentation of the analytical methods and parameters used, ensuring transparency and reproducibility of the results.
Expert Interpretation: Our specialists offer their expert interpretation of the analysis results, highlighting key findings and their implications for your project or research.
Recommendations for Further Research or Development: Based on the analysis outcomes, we provide tailored recommendations for future studies or product development efforts involving eriodictyol.
Applications of Eriodictyol Analysis
Molecular Biology and Genetics: Eriodictyol analysis elucidates its molecular mechanisms, revealing how it influences gene expression and protein function. This insight is crucial for understanding its potential in modulating biological pathways involved in cell growth and differentiation.
Pharmacology and Toxicology: Determining eriodictyol's pharmacodynamics and pharmacokinetics through analysis informs its therapeutic viability and safety. This research is vital for uncovering possible drug interactions and side effects.
Nutritional Science: Analysis quantifies eriodictyol in food and studies its bioavailability, guiding research on its dietary benefits and role in disease prevention, particularly for metabolic and cardiovascular diseases.
Plant Science: Eriodictyol analysis in plants helps in understanding flavonoid biosynthesis, affecting breeding and cultivation strategies to optimize flavonoid content. It also sheds light on flavonoids' ecological roles.
Biochemistry and Chemical Ecology: Studying eriodictyol's biochemical properties and its ecological interactions offers insights into its roles in plant defense and the ecological implications of flavonoid production.
Case. Comprehensive Analysis of Thonningia sanguinea Phytoconstituents: Integration of Extraction, GC-MS/MS, and Molecular Docking Techniques
Background
Thonningia sanguinea, a widely used medicinal herb in tropical Africa, exhibits diverse pharmacological properties. Its bioactive compounds have shown significant antimicrobial, antioxidant, and anticancer effects. This study aimed to develop a comprehensive analytical approach to quantify and characterize key phytoconstituents in T. sanguinea.
Sample
T. sanguinea plants were collected from Ghana, and their crude extract and ethyl acetate fraction were prepared for analysis. Five isolated compounds, including epipinoresinol, β-sitosterol, eriodictyol, betulinic acid, and secoisolariciresinol, were investigated.
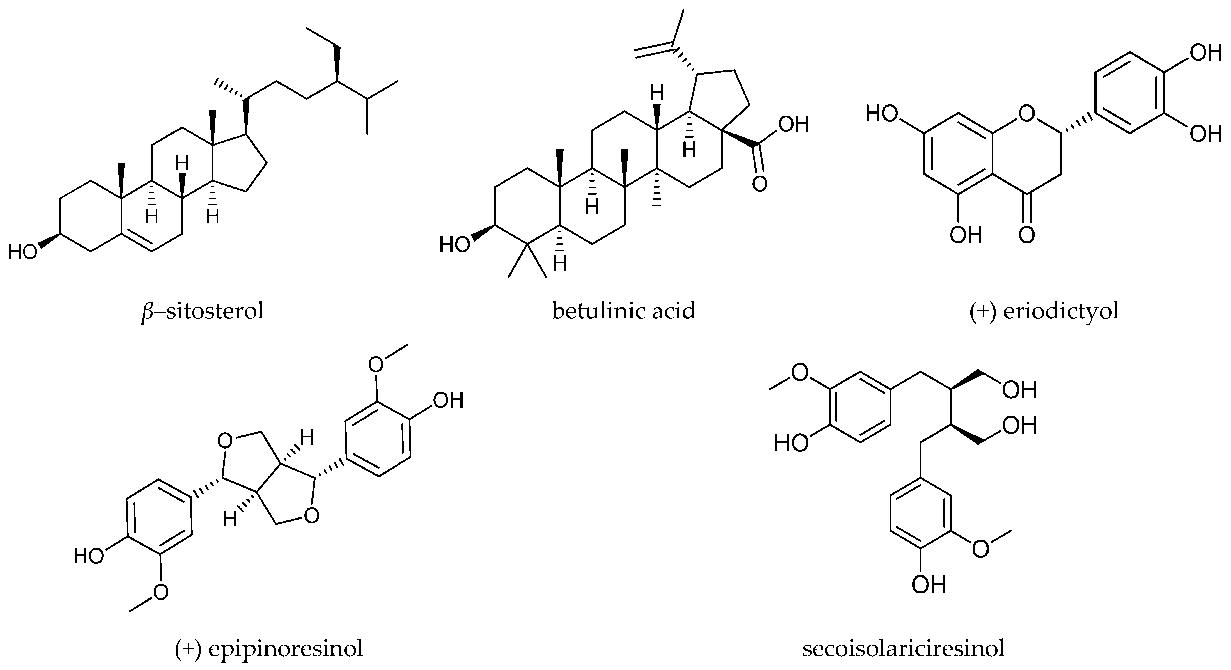 Structures of the analyzed compounds.
Structures of the analyzed compounds.
Technical Methods
Extraction and Fractionation: T. sanguinea plants were dried, extracted with methanol, and fractionated with organic solvents to obtain crude extract and fractions of different polarities.
Isolation of Pure Compounds: Pure compounds were isolated from the extract using standard procedures and characterized via NMR spectroscopy.
Gas Chromatography Coupled to Tandem Mass Spectrometry (GC-MS/MS): A validated GC-MS/MS method was developed to quantify the isolated compounds. Calibration curves were constructed, and plant samples were assayed under optimized conditions.
Molecular Docking Studies: The virtual binding of isolated compounds to the epidermal growth factor receptor (EGFR) active site was assessed using molecular docking simulations. Binding energies and ligand-receptor interactions were analyzed.
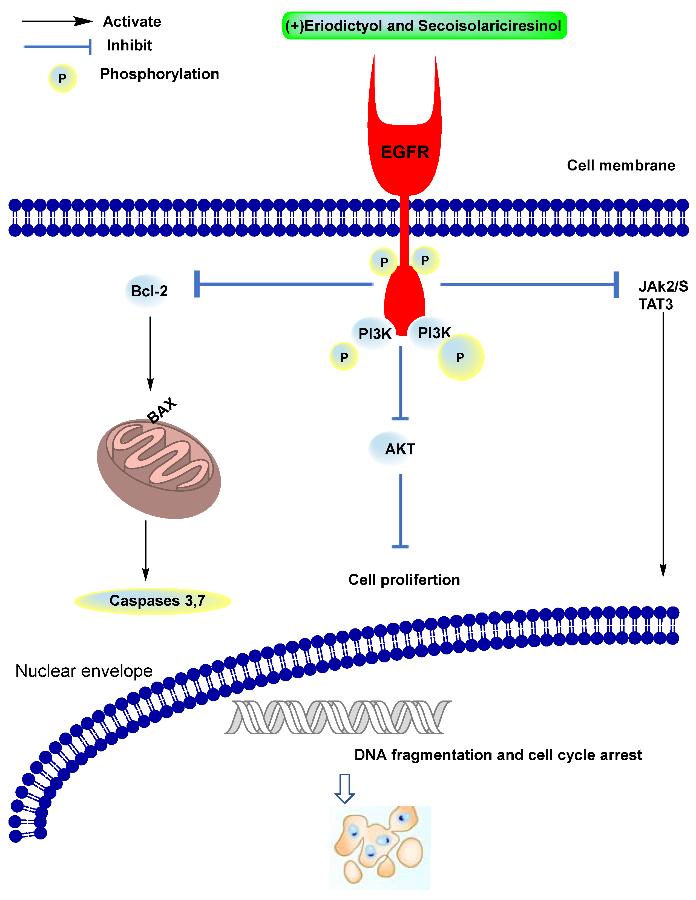 Schematic diagram of the EGFR-based pathway of anticancer activity.
Schematic diagram of the EGFR-based pathway of anticancer activity.
Results
The developed GC-MS/MS method exhibited excellent linearity, sensitivity, precision, and accuracy for quantifying the target compounds. Analysis of T. sanguinea samples revealed varying concentrations of phytoconstituents in the crude extract and ethyl acetate fraction. Molecular docking studies demonstrated the potential of identified compounds to inhibit EGFR, suggesting their role as cancer therapeutic agents.
Reference
- Elhady, Sameh S., et al. "GC-MS/MS Quantification of EGFR Inhibitors, β-Sitosterol, Betulinic Acid,(+) Eriodictyol,(+) Epipinoresinol, and Secoisolariciresinol, in Crude Extract and Ethyl Acetate Fraction of Thonningia sanguinea." Molecules 27.13 (2022): 4109.


 Eriodictyol
Eriodictyol Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service Structures of the analyzed compounds.
Structures of the analyzed compounds. Schematic diagram of the EGFR-based pathway of anticancer activity.
Schematic diagram of the EGFR-based pathway of anticancer activity.

