What are Alkaloids?
Alkaloids represent a vast and diverse group of secondary metabolites found in plants, animals, and microorganisms. These compounds are typically characterized by the presence of one or more nitrogen atoms within a cyclic structure. Alkaloids exhibit a wide range of chemical properties, with varying solubilities, colors, and tastes. They can be classified into several major groups based on their chemical structures, including pyrrolidines, pyridines, piperidines, tropanes, indoles, and isoquinolines, among others. Tannins are polyphenols with astringent properties found in various plant species. They have been traditionally used in the tanning process for animal hides and are known for their ability to bind proteins. Tannins are further classified into hydrolyzable tannins and condensed tannins, each with its own chemical structure and biological activities.
Alkaloids often possess significant biological activities such as antibacterial, antiviral, anticancer and analgesic properties. The study of alkaloids can lead to the discovery of novel drug candidates and therapeutic agents. Secondly, alkaloid analysis plays a crucial role in the identification and certification of medicinal plants and herbal products to ensure their quality and safety. In addition, alkaloids contribute to the chemical diversity and ecological roles of organisms, and alkaloid analysis is therefore essential for understanding the intricate relationships within ecosystems.
Alkaloids Analysis at Creative Proteomics
1. Alkaloid Metabolomics Profiling
By combining liquid chromatography (LC) and mass spectrometry (MS), Creative Proteomics enables the sensitive and accurate detection, identification, and quantification of alkaloids in complex biological samples. We utilize advanced instruments such as the Thermo Scientific Q Exactive Plus Hybrid Quadrupole-Orbitrap Mass Spectrometer to achieve high-resolution metabolite profiling.
2. Alkaloid Structural Elucidation
Creative Proteomics employs a range of techniques to determine the precise chemical structure of alkaloids. Nuclear magnetic resonance (NMR) spectroscopy coupled with mass spectrometry is used to elucidate the connectivity and stereochemistry of alkaloid molecules, contributing to the understanding of their biological activity and potential pharmacological applications.
3. Alkaloid Quantitative Analysis
Quantitative analysis of alkaloids is essential for studying their distribution, metabolism, and bioavailability. Creative Proteomics offers accurate and precise quantification of alkaloids in various sample matrices, including plant extracts, animal tissues, and cell cultures. Utilizing stable isotope-labeled internal standards and validated analytical methods, we ensure reliable and reproducible results.
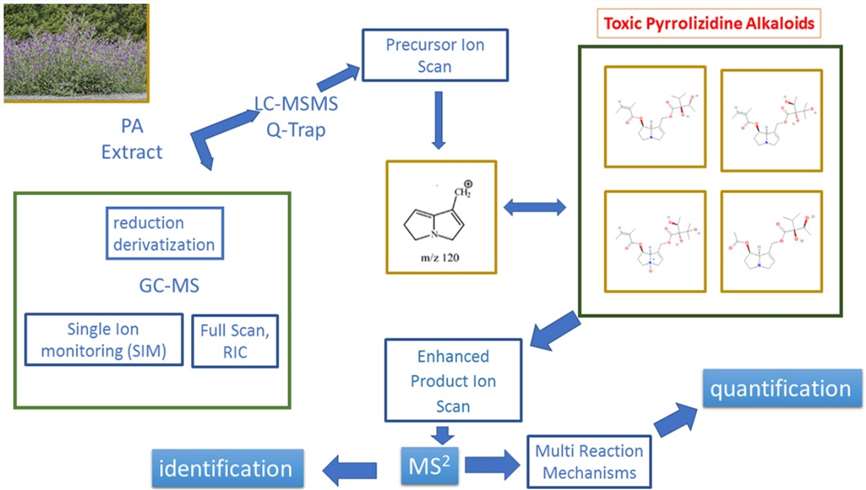
 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service
List of Alkaloids Analyzed (including but not limited to)
| Types |
Compounds |
| Pyrrolidines |
Nicotine, Lobeline, Anabasine, Pseudooxynicotine |
| Indoles |
Tryptamine, Serotonin, Psilocybin, Ibogaine |
| Isoquinolines |
Morphine, Papaverine, Noscapine, Sanguinarine |
| Tropanes |
Atropine, Cocaine, Scopolamine, Hyoscyamine |
| Quinolizidines |
Lupinine, Cytisine, Sparteine, Anagyrine |
| Pyridines |
Anabasamine, Anabaseine, Anatabine, Myosmine |
| Purines |
Caffeine, Theobromine, Theophylline, Xanthine |
| Imidazoles |
Histamine, Naphazoline, Imidazolethylamine, Clonidine |
| Benzylisoquinolines |
Papaveraldine, Reticuline, Norreticuline, Berberine |
Why Choose Us?
- Detection platform: Untargeted metabolome + targeted metabolome detection technology for metabolite detection, taking into account both qualitative and quantitative accuracy.
- Self-constructed database: Construct an alkaloid metabolite database independently and provide all standard curves of the measured substances.
- Strict QC: Standard QC+Sample QC dual QC system + exogenous internal standard to ensure data stability/accuracy.
- Accurate characterization: Each compound contains more than two pairs of characteristic ions, one pair is used as quantitative ion pair, and the other pairs are used to assist the characterization, and RT confirmation, to ensure the accuracy of the description.
- Accurate quantification: Triple quadrupole MRM mode, ESI ion source, the gold standard for quantification, precise detection of the relative content of substances.
- Broad linear range: Correlation coefficient: >0.99 to meet the quantitative analysis of different types of complex samples.
- Manual calibration: Strict manual calibration process ensures the accuracy and reliability of the method.
Sample Requirements for Alkaloids Metabolism Assay
| Sample Types |
Minimum Sample Size |
Biological Repeat |
| Plant Samples |
Roots, stems and leaves, flower parts, fruits/seeds, rhizomes, buds/tender leaves, tissue sections, pollen, bark, trunk/wood, resin/gum, resin acids, seedlings/saplings, rhizosphere soil |
600mg |
3-6 |
| Liquid Samples |
Root secretions |
10mL |
| Fermentation fluid, wine, tissue fluid, fruit juice |
5mL |
| Honey, nectar, oil, extracts |
500uL |
| Specialty Samples |
Cultured samples, presence of liquids |
600mg |
Case 1. GC-MS and LC-MS/MS Analysis of Alkaloid Metabolites in Echium plantagineum: Insights into Characterization and Quantification
Background:
Pyrrolizidine alkaloids are a diverse class of compounds with various biological activities. Understanding their metabolism is important for assessing their toxicity and developing analytical methods for their detection. Analyzing the metabolites of pyrrolizidine alkaloids provides insights into their fate and distribution within organisms, aiding in risk assessment and control strategies.
Sample:
In the study, the samples used for alkaloid metabolite analysis were the aerial parts of Echium plantagineum L., a plant from the Boraginaceae family. Two extracts were obtained: one from air-dried plant material and another from fresh aerial parts of the plant. These extracts served as the basis for further analysis of alkaloid metabolites.
Technical Platform and Procedure:
GC-MS analysis was conducted using a Hewlett Packard 6890 GC coupled with a single quadrupole mass spectrometer detector HP 5973. The GC column used was HP-5MS with specific dimensions. The analysis was performed under electron impact source (EI) mode, and the NIST 11 spectral library was utilized for spectral comparison.
LC-MS/MS analysis was carried out using a 1260 Infinity HPLC system coupled with a 4000 QTRAP® System mass spectrometer. The HPLC system was equipped with a Zorbax Eclipse XDB C18 column. The analysis utilized electrospray ionization (ESI) as the ion source, operating in positive ion mode. The mobile phase consisted of specific compositions of formic acid and acetonitrile. The LC-MS/MS analysis involved multiple ion detection in the first quadrupole (Q1 MI) with various scan modes, such as enhanced product ion scan (EPI), precursor ion scan (PIS), and multiple reaction monitoring (MRM). Optimized conditions were employed based on acetyl lycopsamine and the extracts obtained.
Sample Preparation:
For GC analysis, the organic extract obtained from the Echium plantagineum samples was reconstituted in dry chloroform, followed by the addition of bis-trimethylsilylacetamide. The mixture was stirred under specified conditions.
For LC-MS/MS analysis, the extracts obtained from Echium plantagineum were prepared and reconstituted in a suitable solvent before injection into the LC system.
Analytical Procedures:
GC-MS analysis involved full scan acquisition with spectral library comparison and selected ion monitoring (SIM) mode. The mass spectra were recorded at a specific electron energy level.
The extracts obtained from Echium plantagineum were analyzed using liquid chromatography coupled to mass spectrometry. ESI source was employed in positive ion mode, and multiple ions detection in the first quadrupole was performed using various scan modes.
Results
The analysis of alkaloid metabolites using the mentioned techniques yielded significant results. GC-MS analysis identified specific alkaloid compounds in the organic extract based on characteristic mass spectral data. With LC-MS/MS analysis, various pyrrolizidine alkaloids and their N-oxides were identified and quantified in the plant extracts.
Additionally, LC-MS/MS made it easier to characterize a pyrrolizidine alkaloid derivative that was previously unidentified. Precursor ion scan (PIS) tests were used to identify and measure certain alkaloid ions. These discoveries shed important light on the main alkaloid species, their concentrations, and metabolic processes.
The LC-MS/MS workflow involved a combination of PIS and enhanced product ion (EPI) experiments, allowing researchers to select specific ions and fragmentation pathways for alkaloid identification and quantification. This approach proved highly effective in characterizing alkaloid metabolites and standardizing their content.
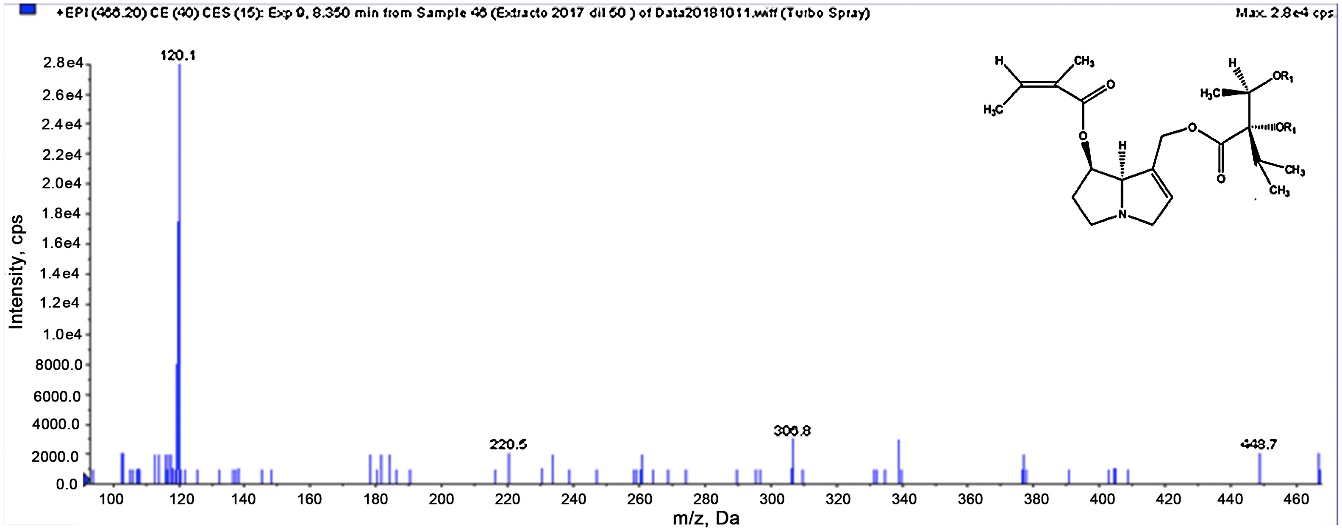 Proposed structure and EPI experiment of m/z 466 compound (R1 is indistinctly either H or C5H7O2 groups).
Proposed structure and EPI experiment of m/z 466 compound (R1 is indistinctly either H or C5H7O2 groups).
Reference
- Sixto, Alexandra, et al. "GC–MS and LC–MS/MS workflows for the identification and quantitation of pyrrolizidine alkaloids in plant extracts, a case study: Echium plantagineum." Revista Brasileira de Farmacognosia 29 (2019): 500-503.


 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service
 Proposed structure and EPI experiment of m/z 466 compound (R1 is indistinctly either H or C5H7O2 groups).
Proposed structure and EPI experiment of m/z 466 compound (R1 is indistinctly either H or C5H7O2 groups).

