What is Prunus persica (Peach)?
Prunus persica, commonly known as the peach, is a beloved and widely cultivated fruit celebrated for its exquisite taste and unique flavor. Beyond its delectable nature, peaches conceal a rich tapestry of metabolic intricacies, presenting a compelling subject for metabolomics analysis.
Metabolomics analysis of peaches is a powerful scientific tool aimed at delving deep into the biochemical metabolism of these fruits. This field focuses on the analysis and interpretation of various metabolites present in peaches, including vitamins, minerals, antioxidants, and bioactive molecules, all of which are crucial factors influencing peach characteristics and nutritional value.
The methods employed in metabolomics analysis of peaches encompass a wide array of techniques and tools for dissecting and quantifying the metabolites present in peaches. These methods include, but are not limited to, mass spectrometry, nuclear magnetic resonance, liquid chromatography, and gas chromatography, all highly precise technologies. Through these methods, we can identify, quantify, and compare the metabolites in peaches under different growth conditions, unveiling vital information about peach growth, development, and responses to the environment.
Metabolomics analysis of Prunus persica not only provides an avenue for an in-depth understanding of peaches but also establishes a robust foundation for various applications, from improving food quality to advancing agricultural sustainability, and discovering potential bioactive compounds. Partner with Creative Proteomics to harness this scientific tool and unlock the mysteries held within peaches.
Our Prunus persica (Peach) Metabolomics Analysis Projects
- Nutritional Profiling: Our comprehensive analysis allows us to uncover the full nutritional composition of peach fruit. We quantify essential vitamins, minerals, antioxidants, and other bioactive compounds that contribute to its dietary value. Whether you're exploring peach's role in nutrition or seeking to optimize its cultivation for maximum nutritional benefit, our data can guide your research.
- Bioactive Compound Discovery: We specialize in identifying and characterizing bioactive compounds within Prunus persica. These compounds may hold the key to potential health benefits. By delving into the peach metabolome, we can uncover novel compounds with applications in pharmaceuticals, functional foods, or dietary supplements.
- Flavor and Aroma Elucidation: Peach's unique flavor and aroma are what set it apart. Our metabolomics analysis allows us to explore the volatile organic compounds responsible for these sensory attributes. Understanding the chemical basis of peach's sensory appeal is not only critical for breeding but also for consumer preferences and product development.
- Pharmacological Investigations: If you're interested in peach's potential therapeutic properties, our metabolomics services can help. We investigate the role of peach metabolites in promoting human health. This research may lead to the development of new pharmaceuticals or nutraceuticals.
- Crop Improvement: Enhancing peach agriculture is a core focus. By uncovering metabolic markers associated with desirable traits such as improved nutritional content, extended shelf-life, and resistance to environmental stressors, we contribute to the advancement of peach cultivation practices.
- Stress Response Studies: Environmental factors can significantly impact peach trees. Our research examines how peach metabolites respond to various environmental stresses, including drought, disease, and temperature fluctuations. These insights offer a deeper understanding of peach's adaptability and resilience.
- Post-Harvest Handling: Post-harvest handling plays a crucial role in peach quality. Our metabolomics analysis tracks metabolite changes during storage and transportation, providing strategies to extend shelf life and reduce food waste.
- Molecular Breeding: For breeding programs aiming to create superior peach varieties, we identify metabolic markers linked to desirable traits such as disease resistance, improved fruit size, and enhanced coloration.
- Eco-Physiological Studies: Explore how peach metabolites contribute to the tree's response to environmental factors. This knowledge is vital for sustainable orchard management and conservation efforts.
- Quality Control and Authentication: Develop metabolomic profiles for quality control and authentication of peach products, ensuring consumers receive genuine and high-quality peach-based goods.
- Microbiome Interactions: Investigate how the peach metabolome interacts with the microbiome of the fruit, soil, and rhizosphere, uncovering potential applications in agriculture and human health.
- Phytochemical Profiling: Our analysis extends to the characterization of secondary metabolites, such as phenolics, flavonoids, and polyphenols, shedding light on their roles in plant defense mechanisms and human health.
- Environmental Impact Assessment: Explore how environmental factors, like soil composition and water quality, influence the metabolite profiles of peach trees. This research can guide sustainable agriculture practices.
- Metabolite Transport and Storage: Study the transport and storage of peach metabolites within the plant to inform nutrient management and storage preservation strategies.
- Metabolic Signaling: Investigate the role of peach metabolites in intercellular and intracellular signaling processes, providing insights into growth, development, and stress responses.
- Metabolomics in Food Technology: Utilize peach metabolomics data to develop innovative peach-based food products, including beverages, snacks, and functional foods, capitalizing on the fruit's unique bioactive compounds.
- Metabolomics in Biotechnology: Apply peach metabolomics data for biotechnological applications, such as metabolic engineering of other crops to enhance nutritional content or stress tolerance.
Prunus persica (Peach) Metabolomics Analysis Techniques
Liquid Chromatography-Mass Spectrometry (LC-MS): LC-MS is a versatile technique for separating and quantifying metabolites in peach samples. High-resolution instruments like the Thermo Scientific Q Exactive HF-X or the Waters Xevo G2-XS QTof offer precise mass measurements and exceptional sensitivity.
Gas Chromatography-Mass Spectrometry (GC-MS): GC-MS is well-suited for analyzing volatile compounds in peaches. Instruments such as the Agilent 7890A GC System coupled with the Agilent 5977A MSD provide excellent separation and detection capabilities.
Direct Infusion Mass Spectrometry (DIMS): DIMS is a direct approach to analyze polar and non-polar metabolites in peach samples. Instruments like the Bruker maXis Impact and the Thermo Scientific Orbitrap Fusion are capable of high-resolution direct infusion analysis.
Tandem Mass Spectrometry (MS/MS): MS/MS techniques, such as Triple Quadrupole Mass Spectrometry, are employed for targeted analysis of specific metabolites or classes of compounds within peaches. The AB Sciex Triple Quad 6500 is an example of a state-of-the-art triple quadrupole mass spectrometer.
 Workflow for Metabolomics Service
Workflow for Metabolomics Service
Sample Requirements for Peach Metabolomics
| Sample Type |
Sample Size |
Sample Preparation |
| Peach Fruit Tissue |
50-100 grams |
Freeze-dry and powder |
| Peach Leaves |
50-100 grams |
Freeze-dry and powder |
| Peach Roots |
50-100 grams |
Freeze-dry and powder |
| Peach Pulp Juice |
5-10 milliliters |
Centrifuge and filter |
| Peach Skin |
50-100 grams |
Freeze-dry and powder |
| Peach Seeds |
10-20 grams |
Freeze-dry and powder |
| Peach Blossoms |
10-20 grams |
Freeze-dry and powder |
| Peach Wood |
Small wood segments |
Freeze-dry and powder |
| Soil Samples |
100-200 grams (topsoil) |
Air-dry, grind, and sieve |
| Water Samples |
100-200 milliliters |
Filter and store in the dark |
Case. Metabolomics-Based Assessment of Botanical Extracts and Pesticide Residue Fate in Fruit Matrices
Background
The study focuses on utilizing untargeted metabolomics, specifically the Exometabolomic Framework (EMF), to investigate the fate of botanical pesticides (BPs) and chemical pesticides in peach peel samples. BPs and chemical pesticides are commonly used in agriculture to protect crops from pests and diseases. Understanding how these compounds degrade and persist in fruit matrices is essential for pesticide management and food safety.
Samples
The study involves the analysis of peach peel samples subjected to treatments with three different types of pesticides: Akivi-treated samples (botanical pesticide), Armicarb®-treated samples (mineral extract), and Chemical reference-treated samples (chemical pesticides). Untreated control samples were also collected for comparison.
Technological Methods
Exometabolomic Framework (EMF): The EMF is employed to study the fate of pesticide compounds in peach peel samples. It involves several steps:
- Detection and Separation: Pesticide xenometabolites are detected and separated from the endometabolites of peaches.
- Temporal Tracking: The temporal evolution of pesticide xenometabolites is monitored over multiple time points after treatment.
- Statistical Analysis: Statistical methods, including Principal Component Analysis (PCA) and Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA), are used to discern patterns and trends in pesticide degradation and persistence.
Analytical Techniques: High-resolution mass spectrometry is employed to detect and quantify xenometabolites. The analysis includes the use of a C18 Reverse-Phase LC (RPLC) column to separate and identify compounds.
Quantification: Quantification of pesticide active substances (AS) and xenometabolites is performed, and the results are compared to regulatory thresholds to assess pesticide residue levels.
Results
Akivi Treatment: Akivi botanical extract showed a dissipation pattern in the peach samples over time. Some features associated with the extract disappeared, but complete residue dissipation was not observed. PCA analysis revealed distinctions between different time points and treatments.
Armicarb Treatment: While the mineral AS (KHCO3) of Armicarb could not be detected due to its high solubility, the EMF approach successfully identified certain xenometabolites associated with Armicarb. These compounds persisted throughout the study.
Chemical Reference Treatment: The study detected and quantified the ASs of the chemical pesticides in the treated samples, as well as some by-products. Discrimination between treated and untreated samples was possible, despite contamination of the untreated control samples. Persistence of ASs and degradation patterns of by-products were observed.
Contamination Issues: The study highlighted the challenge of contamination in field experiments, particularly through spray drift. This contamination impacted the untreated control samples and should be addressed in future research.
Variability: Field conditions introduced variability in the study, including variations in pesticide exposure and environmental factors. To mitigate this, improvements in sampling strategies and replication are suggested.
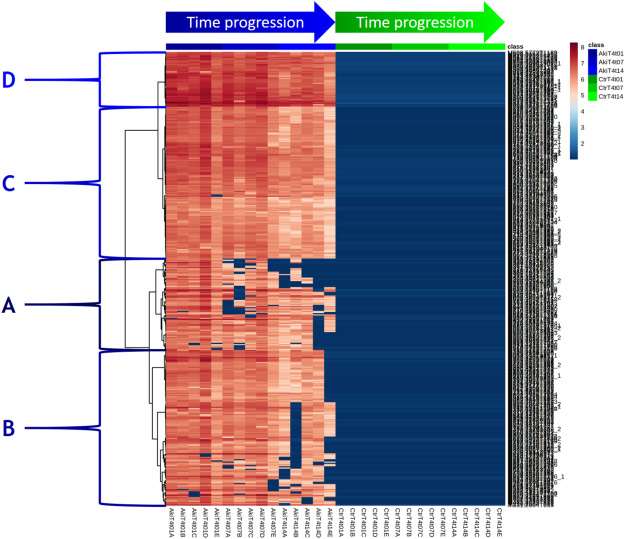 Heatmap of Akivi xenometabolites abundance
Heatmap of Akivi xenometabolites abundance
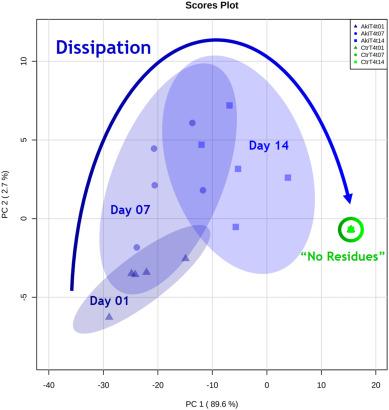 PCA of Akivi xenometabolites degradation kinetics
PCA of Akivi xenometabolites degradation kinetics
Reference
- Ramos, Mélina, et al. "Untargeted metabolomics as a tool to monitor biocontrol product residues' fate on field-treated Prunus persica." Science of the Total Environment 807 (2022): 150717.


 Workflow for Metabolomics Service
Workflow for Metabolomics Service Heatmap of Akivi xenometabolites abundance
Heatmap of Akivi xenometabolites abundance PCA of Akivi xenometabolites degradation kinetics
PCA of Akivi xenometabolites degradation kinetics

