What is Polysaccharide Metabolism?
Polysaccharides are large and complex carbohydrates formed by long chains of monosaccharide units connected by glycosidic bonds. These compounds and their derivatives are found in various parts of plants, including the fruit, roots, stems, and flowers. In plants, polysaccharides play crucial roles such as storing energy, providing structural support, facilitating information storage and recognition, regulating physiological processes, and modulating the immune system.
Polysaccharide metabolism refers to the biochemical processes involved in the synthesis, modification, and degradation of polysaccharides within living organisms. These complex and diverse biomolecules undergo biosynthesis through pathways involving the conversion of sucrose, the transformation of uridine diphosphate (UDP)-glucose to other nucleotide diphosphate (NDP) sugars, and polysaccharide polymerization. At Creative Proteomics, we offer comprehensive services to support research and development in various scientific and industrial fields, providing critical insights into their structure and function.
Polysaccharide Metabolism Analysis Service by Creative Proteomics
Extraction and Purification of Polysaccharides and Polysaccharide Metabolites (Optional): At Creative Proteomics, we begin by pulverizing plant samples for analysis, then extract polysaccharides from the tissues. This is followed by decontamination to remove impurities like proteins, fats, and pigments. The samples are further purified through ion and gel purification techniques, ensuring high-purity polysaccharides are ready for detailed analysis.
Basic Analysis of Polysaccharides and Polysaccharide Metabolites: Our service provides comprehensive polysaccharide and metabolite analysis, encompassing composition analysis, molecular weight distribution detection, and advanced structural analysis. The specific details are as follows:
(1) Composition Analysis
We start by hydrolyzing polysaccharides into monosaccharides using acid hydrolysis. These monosaccharides are then identified and quantified through advanced techniques such as HPLC, GC-MS, IC, and HPAEC-PAD. This ensures precise and detailed composition analysis, allowing for accurate identification of the polysaccharide components.
(2) Molecular Weight Distribution
To assess the molecular weight distribution of polysaccharides, we utilize GPC-RI and GPC-RI-MALS technologies. These methods allow us to determine absolute and average molecular weights, dispersity coefficients, and molecular configurations, providing a thorough understanding of the polysaccharide's molecular characteristics.
(3) Advanced Structural Analysis
Our detailed structural analysis is performed using one-dimensional and two-dimensional nuclear magnetic resonance (NMR) techniques. This advanced analysis reveals the intricate structures and functional properties of polysaccharides, aiding in the elucidation of their complex architectures.
Quantitative Characterization of Polysaccharides and Polysaccharide Metabolites: We use ion chromatography (IC) to quantify polysaccharides and their metabolites, especially soluble sugars like oligosaccharides. IC offers precise quantification and high-resolution separation of different types of oligosaccharides in samples. Additionally, we utilize a high-resolution mass spectrometry platform (UPLC LTQ-Orbitrap MS/MS) for broad-targeted oligosaccharide omics analysis, screening for different oligosaccharide substances to predict and analyze the functions of oligosaccharide substances in the samples.
List of Polysaccharides and Polysaccharide Metabolites (including but not limited to)
| Polysaccharides |
Additional Notes |
| Cellulose |
A linear polymer of glucose units linked by β(1→4) glycosidic bonds, forming the structural component of plant cell walls. |
| Hemicellulose |
A heteropolymer of various sugars, including xylose, mannose, and galactose, found in plant cell walls alongside cellulose. |
| Pectin |
A complex polysaccharide composed of galacturonic acid units, contributing to the structural integrity of plant cell walls and acting as a gelling agent. |
| Starch |
A mixture of amylose and amylopectin, both composed of glucose units, serving as a major energy storage polysaccharide in plants. |
| Lignin |
A complex polymer of phenylpropanoid units, providing structural support and rigidity to plant cell walls. |
Techniques and Instrumentation for Polysaccharide Metabolism Analysis
High-Performance Anion-Exchange Chromatography with Pulsed Amperometric Detection (HPAEC-PAD): Creative Proteomics employs the Dionex ICS-5000+ instrument to conduct precise analysis and quantification of polysaccharide components. This technique enables researchers to identify and quantify various monosaccharides, oligosaccharides, and polysaccharides in complex mixtures.
Gas Chromatography-Mass Spectrometry (GC-MS): GC-MS is particularly effective in analyzing volatile components of polysaccharides, such as sugar alcohols and organic acids. We employ the Agilent 7890A-5975C instrument to facilitate a comprehensive characterization of polysaccharide metabolism. Additionally, our methylation analysis service enables the formation of partially methylated sugar alcohol acetate derivatives, which are crucial for determining the linkage patterns of individual sugar residues.
Nuclear Magnetic Resonance Spectrometry (NMR): Our capabilities extend to NMR spectroscopy, with access to the Varian VNMRS 600 MHz instrument. NMR provides valuable information on the linkage patterns, branching, and conformation of polysaccharides, aiding in the determination of their structural features and functional properties in biological systems.
UPLC LTQ-Orbitrap MS/MS Platform: A high-resolution mass spectrometry platform, which includes the Waters ACQUITY UPLC and AB Sciex Triple TOF 5600+ instruments equipped with an electrospray ionization source, is utilized for targeted omics analysis. This platform is employed to screen for differential polysaccharide substances to predict and analyze the functions of these substances within the samples.
Why Choose Us?
- Custom Extraction and Purification Methods (Optional): Creative Proteomics's polysaccharide analysis service begins with tailored extraction and purification protocols aimed at maximizing the yield and purity of the target polysaccharides. These polysaccharides are subsequently refined through ion purification and gel purification processes, ensuring preparations of high purity suitable for detailed structural and functional analysis.
- Accurate Quantification and Identification: Our advanced instrumentation, including High-Performance Anion-Exchange Chromatography with Pulsed Amperometric Detection (HPAEC-PAD), Gas Chromatography-Mass Spectrometry (GC-MS), Nuclear Magnetic Resonance (NMR) Spectroscopy, and the UPLC LTQ-Orbitrap MS/MS platform, guarantees high precision and accuracy in our analyses. These state-of-the-art tools enable detailed compositional, structural, and functional analysis of polysaccharides and their metabolites.
- Customization and Flexibility: Recognizing the uniqueness of each research project, we offer customizable analysis packages tailored to meet specific research needs. Whether detailed monosaccharide composition analysis or comprehensive structural elucidation is required, our flexible services can be adapted to the project's specifications.
- Comprehensive Data Analysis: Our service encompasses every step of polysaccharide analysis, from extraction and purification to advanced structural characterization. This thorough approach ensures that researchers acquire a complete understanding of their samples, facilitating better-informed decisions and enhancing research outcomes.
Applications of Polysaccharide Metabolism Analysis
Functional Food and Nutraceutical Development: Functional food and nutraceutical development heavily relies on polysaccharides due to their notable bioactive properties, encompassing antioxidant, anti-inflammatory, and immune-modulating effects. Our analytical services specialize in identifying and quantifying these bioactive polysaccharides, thereby fostering the creation of premium health-promoting products.
Traditional Chinese Medicine Research: In the realm of Traditional Chinese Medicine (TCM) research, polysaccharides serve as fundamental constituents within numerous medicinal plants, serving as pivotal markers for both quality control and pharmacological investigations. Our meticulous analysis services are tailored to bolster research endeavors aimed at unraveling the therapeutic potential inherent in these polysaccharides, thus ensuring the steadfastness and efficacy of herbal medicines.
Plant Physiology and Biotechnology: Plant physiology and biotechnology are intrinsically intertwined with the understanding of polysaccharide metabolism. This comprehension is indispensable for delving into plant physiology intricacies and enhancing crop characteristics. Our comprehensive suite of analytical services facilitates in-depth explorations into plant cell wall architecture, energy storage mechanisms, and stress response pathways.
Sample Requirements for Polysaccharides and Polysaccharide Metabolites Assay
| Plant Sample Type |
Sample Volume |
Storage Conditions |
Additional Notes |
| Plant Tissues |
≥ 200 mg |
Store at -80°C |
Harvest fresh tissues or freeze immediately after collection |
| Plant Extracts |
≥ 500 µL |
Store at -80°C |
Centrifuge extracts before storage to remove debris |
| Plant Cell Cultures |
≥ 1 mL |
Store at -80°C |
Centrifuge cell cultures before storage to remove cell debris |
| Dried Medicinal Herbs |
≥ 50 mg |
Store at -80°C |
Samples should be refrigerated or frozen immediately after collection |
| Microalgae Precipitate |
≥ 100 µL |
Store at -80°C |
- |
Each experimental treatment should have more than 6 biological replicates to ensure robust statistical analysis and reliable interpretation of results.
Case . Influence of the Ecological Environment on the Structural Characteristics and Bioactivities of Polysaccharides from Alfalfa (Medicago sativa L.)
Background:
The study investigates how different ecological environments affect the structural characteristics and bioactivities of polysaccharides extracted from alfalfa (Medicago sativa L.). It compares polysaccharides from normal and saline-alkali soils, focusing on their monosaccharide composition, molecular weight, and bioactivities.
The research aims to elucidate the influence of growth conditions on alfalfa polysaccharides to optimize their use in functional foods and bioactive compounds.
Samples:
The alfalfa was dried, pulverized, and soaked in hot water, followed by ethanol precipitation to obtain crude polysaccharides.
Deproteinization was performed using the Sevag method, and the final product was freeze-dried for analysis. This method ensures the isolation of polysaccharides for further study.
Technical methods procedure:
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were used to observe APS microstructures. SEM provided surface images, while TEM examined molecular assembly in well-dispersed APS solutions.
Fourier transform-infrared (FT-IR) and NMR spectroscopy (1H-NMR, 13C-NMR, correlation spectroscopy) were employed to identify functional groups and determine molecular structures of APS.
Gel permeation chromatography with refractive index and multiangle laser light scattering (GPC-RI-MALS) assessed molecular weight and distribution characteristics, calculating number-average, weight-average, Z-average molecular weights, and polydispersity.
Results
SEM and TEM revealed differences in microstructures and molecular assembly among APS from different environments. APS from arid regions showed compact, aggregated structures, while those from humid areas exhibited loose, dispersed assemblies.
FT-IR and NMR spectra indicated consistent functional groups across APS samples, including hydroxyl, carbonyl, and glycosidic bonds. However, slight variations in signal intensities suggested environmental influence on polysaccharide structures.
GC-MS analysis of methylated APS demonstrated diverse glycosidic linkages, with varying proportions of (1→4)-, (1→3)-, and (1→6)-linked glucose units, reflecting ecological impacts on polysaccharide assembly.
GPC-RI-MALS results showed significant differences in molecular weight distribution and polydispersity among APS samples. Polysaccharides from arid environments had higher molecular weights and narrower distribution, whereas those from humid regions exhibited lower weights and broader distribution.
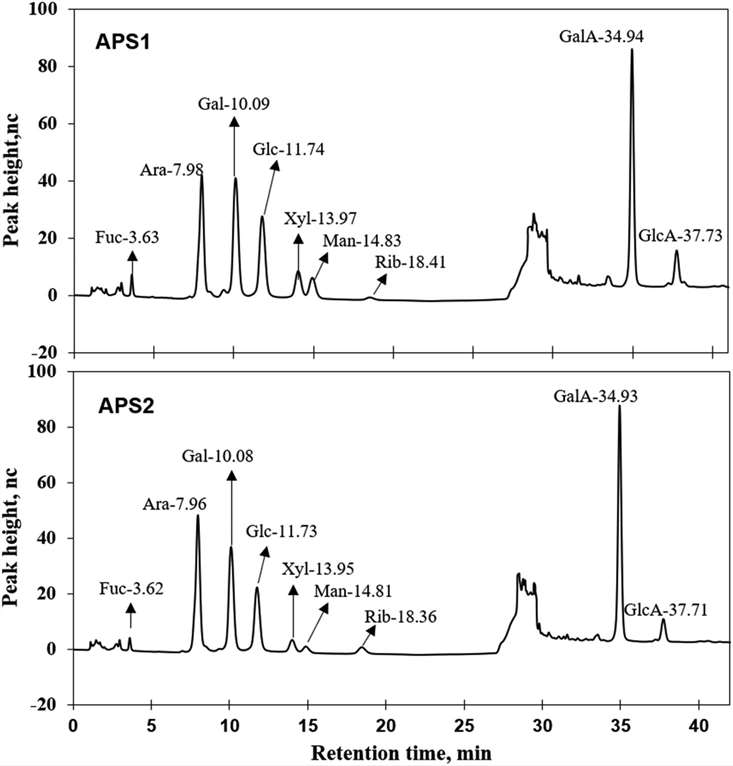 Fig 1. Ion chromatograms of APS1 and APS2. Each absorption peak was annotated in monosaccharide name and retention time.
Fig 1. Ion chromatograms of APS1 and APS2. Each absorption peak was annotated in monosaccharide name and retention time.
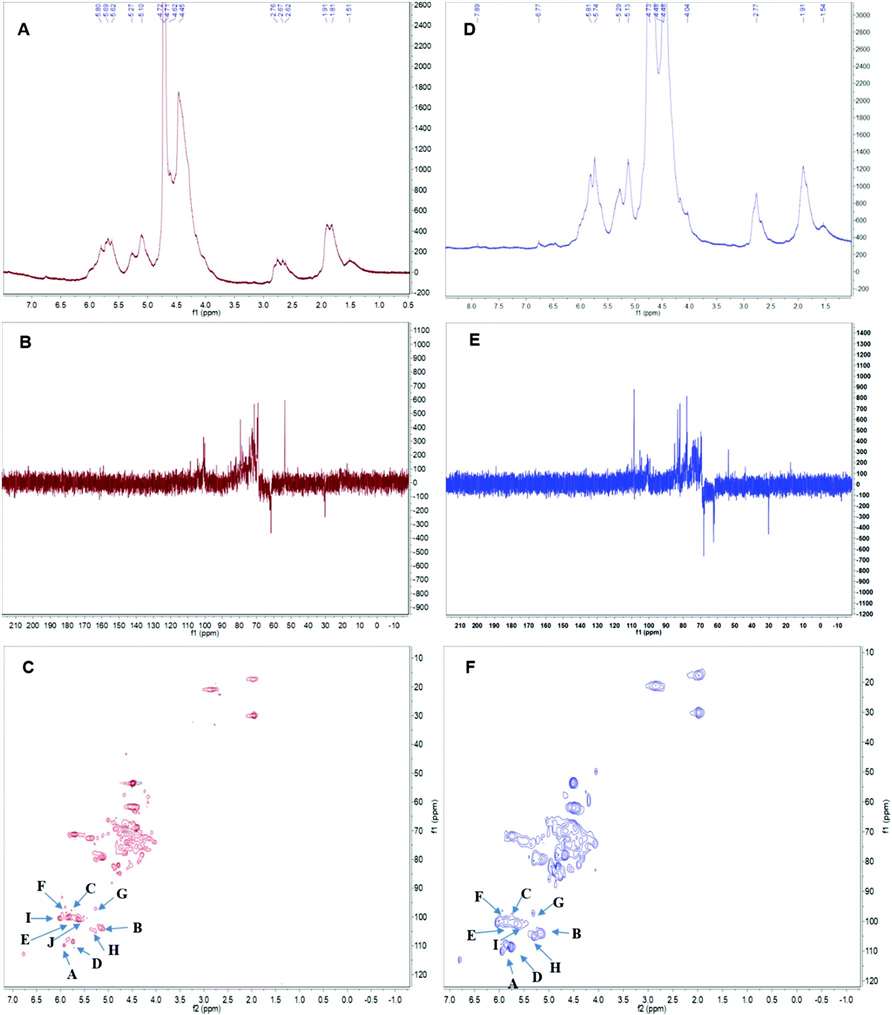 Fig 2. The nuclear magnetic resonance (NMR) spectrum of APS in D2O solution at 25 °C. Panels A & D show the 1H spectra of APS1 and APS2.Panels B & E depict the 13C spectra of APS1 and APS2. Panels C & F depict the heteronuclear multiple bond coherence (HMBC) spectrum of APS1 and APS2.
Fig 2. The nuclear magnetic resonance (NMR) spectrum of APS in D2O solution at 25 °C. Panels A & D show the 1H spectra of APS1 and APS2.Panels B & E depict the 13C spectra of APS1 and APS2. Panels C & F depict the heteronuclear multiple bond coherence (HMBC) spectrum of APS1 and APS2.
Reference
- Zhang, C. (2022). "Influence of the ecological environment on the structural characteristics and bioactivities of polysaccharides from alfalfa (Medicago sativa L.)." Food & Function 13(13), 7029-7045.



 Fig 1. Ion chromatograms of APS1 and APS2. Each absorption peak was annotated in monosaccharide name and retention time.
Fig 1. Ion chromatograms of APS1 and APS2. Each absorption peak was annotated in monosaccharide name and retention time. Fig 2. The nuclear magnetic resonance (NMR) spectrum of APS in D2O solution at 25 °C. Panels A & D show the 1H spectra of APS1 and APS2.Panels B & E depict the 13C spectra of APS1 and APS2. Panels C & F depict the heteronuclear multiple bond coherence (HMBC) spectrum of APS1 and APS2.
Fig 2. The nuclear magnetic resonance (NMR) spectrum of APS in D2O solution at 25 °C. Panels A & D show the 1H spectra of APS1 and APS2.Panels B & E depict the 13C spectra of APS1 and APS2. Panels C & F depict the heteronuclear multiple bond coherence (HMBC) spectrum of APS1 and APS2.

