What is Monosaccharide Metabolism?
Monosaccharides, which are the basic building blocks of carbohydrates, generally containing 3-7 carbon atoms. The carbon backbone contains many hydrophilic groups and is easily soluble in water. They constitute the basic structural units of organisms, and they are also the direct energy supply substances for metabolic activities.
Monosaccharide metabolism refers to the biochemical processes involved in the utilization, interconversion, and regulation of these single sugar molecules within living organisms. These processes encompass glycolysis, gluconeogenesis, the pentose phosphate pathway, the tricarboxylic acid cycle (TCA), and other metabolic pathways. Monosaccharide metabolism is intricately linked to energy production, carbohydrate storage, and the synthesis of complex biomolecules. By studying the metabolic pathways and dynamic accumulation changes of monosaccharides, Creative Proteomics can explore its impact on plant growth and development, stress resistance, and crop quality.
Monosaccharide Metabolism Analysis Service by Creative Proteomics
Monosaccharide Metabolism Qualitative and Quantitative Characterization: In our service, we employ cutting-edge methodologies such as high-performance anion-exchange chromatography with pulsed amperometric detection (HPAE-PAD), Ultra-performance liquid chromatography/multiple reaction monitoring mass spectrometry (UPLC-MRM/MS), and gas chromatography-mass spectrometry (GC-MS) to conduct comprehensive qualitative and quantitative analyses of monosaccharide components. These sophisticated techniques allow for the accurate identification and quantification of individual monosaccharides, providing valuable insights into their spatial distribution and relative abundance within plant samples.
Profile of Central Metabolism: our monosaccharide metabolism analysis service provides a comprehensive profile of central metabolism, encompassing key pathways such as glycolysis, the pentose-phosphate shunt, the tricarboxylic acid (TCA) cycle, nucleotide pools, and NAD-related metabolites. Through the analysis of monosaccharides and their metabolites using UPLC-MRM/MS and GC-MS methods, we offer detailed insights into the dynamics of these metabolic pathways, elucidating their regulation and interconnection within the plant metabolic network.
List of Monosaccharides and Monosaccharide Metabolites (including but not limited to)
| Monosaccharides |
Abbreviation |
| Glucose |
Glc |
| Fructose |
Fru |
| Galactose |
Gal |
| Mannose |
Man |
| Ribose |
Rib |
| Xylose |
Xyl |
| Arabinose |
Ara |
| Rhamnose |
Rha |
| Xylulose |
Xylu |
| Fucose |
Fuc |
Techniques and Instrumentation for Monosaccharide Metabolism Analysis
Liquid Chromatography-Mass Spectrometry (LC-MS): Creative Proteomics utilize the Waters ACQUITY UPLC and AB Sciex TripleQuad 5500 instruments to achieve efficient analysis of monosaccharide metabolism. This method enables precise qualitative and quantitative analysis of various monosaccharides and their metabolites, providing comprehensive analytical insights.
Gas Chromatography-Mass Spectrometry (GC-MS): We also offer Agilent 7890A-5975C instrument for the analysis of monosaccharides and their metabolites. GC-MS technology offers excellent selectivity and sensitivity, enabling effective differentiation of isomers in samples and providing more accurate data for your research.
High-Performance Anion-Exchange Chromatography with Pulsed Amperometric Detection (HPAE-PAD): We choose Dionex ICS-5000+ instrument as the technical platform and HPAE-PAD method to analyze plant monosaccharides and their metabolites. This method allows for efficient analysis of monosaccharide composition without the need for complex derivatization steps, offering simplicity of operation and high resolution.
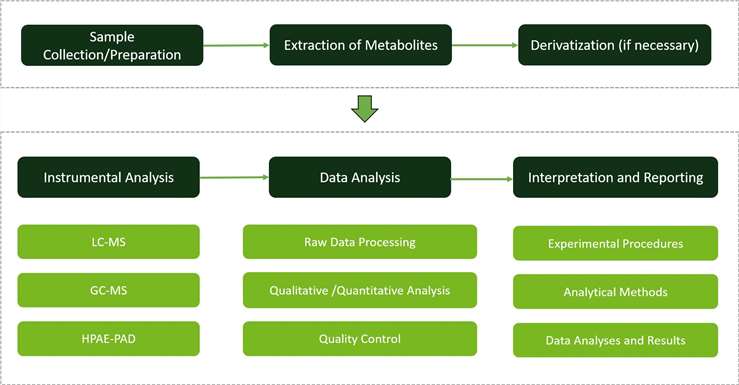 Workflow of Monosaccharide Metabolism Analysis Service
Workflow of Monosaccharide Metabolism Analysis Service
Why Choose Us?
- Pre-Derivatization Processing (Optional): Our comprehensive monosaccharide metabolism analysis service at Creative Proteomics offers the option of pre-derivatization processing, ensuring optimal sample preparation tailored to your specific requirements. This step enhances the accuracy and sensitivity of our analysis, facilitating precise identification and quantification of monosaccharide components.
- Accurate Quantification and Identification: Creative Proteomics utilizes standard reference materials for both qualitative and quantitative analysis of monosaccharides. By employing validated analytical methods and state-of-the-art instrumentation, we ensure accurate determination of monosaccharide concentrations in your samples.
- Customized Analysis Tailored to Specific Research Needs: At Creative Proteomics, we understand that each research project is unique. Therefore, we offer customized monosaccharide metabolism analysis tailored to your specific research needs. Whether you require specialized sample preparation techniques, targeted analysis of specific monosaccharides, or customized reporting formats, our team of experts will work closely with you to design an analysis strategy that meets your exact requirements.
- Comprehensive Data Analysis:Our monosaccharide metabolism analysis service at Creative Proteomics includes comprehensive data analysis to provide valuable insights into your research. This involves employing advanced techniques for raw data pre-processing, sophisticated multivariate analysis methods, and biological annotation.
Applications of Monosaccharide Metabolism Analysis
Quantitative Detection of Plant Monosaccharides: Creative Proteomics's monosaccharide metabolism analysis service provides valuable information on the composition and dynamics of monosaccharides in plants.
Regulation Pathways of Plant Sugar Metabolism: Our analysis service offers insights into the intricate regulatory mechanisms governing sugar metabolism pathways.
Enhancement of Plant Fruit Quality: At Creative Proteomics, we aids in assessing the monosaccharide composition of fruits, such as glucose, sucrose, and fructose, which directly impact taste, aroma, and nutritional value.
Regulation of Plant Growth and Development: By uncovering these regulatory pathways, researchers can devise strategies to manipulate sugar metabolism to optimize plant growth, improve crop yield, and enhance stress tolerance.
Sample Requirements for Monosaccharides and Monosaccharide Metabolites Assay
| Plant Sample Type |
Sample Volume |
Storage Conditions |
Additional Notes |
| Plant Tissues |
≥ 100 mg |
Store at -80°C |
Harvest fresh tissues or freeze immediately after collection |
| Plant Extracts |
≥ 500 µL |
Store at -80°C |
Centrifuge extracts before storage to remove debris |
| Plant Cell Cultures |
≥ 1 mL |
Store at -80°C |
Centrifuge cell cultures before storage to remove cell debris |
| Plant Sap |
≥ 100 µL |
Store at -80°C |
Collect sap using sterile techniques and avoidcontamination |
| Plant Seeds |
≥ 50 mg |
Store at -20°C |
Remove husks or shellsbefore analysis |
Each experimental treatment should have more than 6 biological replicates to ensure robust statistical analysis and reliable interpretation of results.
Case 1. Deciphering the impact of specific sugar metabolites accumulation on the growth and development of pinus massoniana seedlings: a transcriptomic combined with targeted metabolomic approach
Background:
This study delves into the molecular mechanisms governing heteroblastic transitions in P. massoniana seedlings, with a specific focus on the regulation of sugar metabolism.
The objective is to unravel the regulatory pathways of sugar metabolism amidst heteroblastic transitions in pine seedlings by elucidating the metabolic and transcriptional dynamics linked to sugar metabolism regulation during the initial phases of heteroblastic transition.
Samples:
The seeds originated from P. massoniana orchards at Duyun Ma'anshan State-owned Forest Farm, China.
After disinfection, they were germinated and grown under controlled conditions in an illumination incubator for subsequent sampling.
Technical methods procedure:
Pine seedlings at different developmental stages were selected for analysis. Leaf samples were collected from seedlings with primary needles(PN) and those with developing secondary needle buds(SNB).
Sugar metabolites were extracted from the samples using a methanol:isopropanol solution, followed by derivatization and GC-MS analysis.
Sugar contents were quantified using standard curves, and differential metabolite profiles were analyzed using statistical tests and partial least squares-discriminant analysis.
Results
The study investigated carbohydrate-related metabolite variations in primary needle (PN) and secondary needle bud (SNB) seedlings of P. massoniana.
Gas chromatography tandem mass spectrometry (GC‒MS) identified 12 carbohydrates, with PN and SNB seedlings exhibiting distinct carbohydrate profiles. Sucrose (Suc), glucose (Glc), and fructose (Fru) were predominant, with SNB seedlings showing reduced levels compared to PN seedlings.
Transcriptomic analysis revealed 103 DEGs associated with starch and sucrose metabolism pathways. Coexpression analysis identified correlations between carbohydrate contents and gene expression levels.
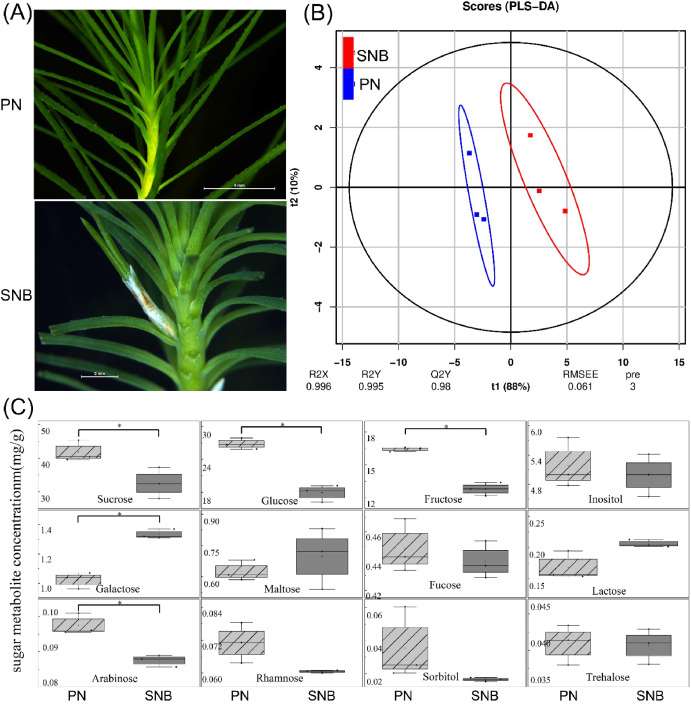 Appearance of PN and SNB seedlings and identification of differential sugar metabolites. (A) The secondary needle buds began to grow from the axillae of the primary needles in SNB seedlings. (B) PLS-DA scores derived from all sugar metabolites detected in three replicate samples of the two types of seedlings. (C) Differences in sugar metabolites of the PN and SNB seedlings; P < 0.05 and VIP≥1, tag: *.
Appearance of PN and SNB seedlings and identification of differential sugar metabolites. (A) The secondary needle buds began to grow from the axillae of the primary needles in SNB seedlings. (B) PLS-DA scores derived from all sugar metabolites detected in three replicate samples of the two types of seedlings. (C) Differences in sugar metabolites of the PN and SNB seedlings; P < 0.05 and VIP≥1, tag: *.
Reference
- Zhao, Y. (2023). "Transcriptomic and targeted metabolomic unravelling the molecular mechanism of sugar metabolism regulating heteroblastic changes in Pinus massoniana seedlings." Plant Physiology and Biochemistry 203, 108029.


 Workflow of Monosaccharide Metabolism Analysis Service
Workflow of Monosaccharide Metabolism Analysis Service Appearance of PN and SNB seedlings and identification of differential sugar metabolites. (A) The secondary needle buds began to grow from the axillae of the primary needles in SNB seedlings. (B) PLS-DA scores derived from all sugar metabolites detected in three replicate samples of the two types of seedlings. (C) Differences in sugar metabolites of the PN and SNB seedlings; P < 0.05 and VIP≥1, tag: *.
Appearance of PN and SNB seedlings and identification of differential sugar metabolites. (A) The secondary needle buds began to grow from the axillae of the primary needles in SNB seedlings. (B) PLS-DA scores derived from all sugar metabolites detected in three replicate samples of the two types of seedlings. (C) Differences in sugar metabolites of the PN and SNB seedlings; P < 0.05 and VIP≥1, tag: *.

