What is Glycine Metabolism?
Glycine, a non-essential amino acid, plays a vital role in plant physiology. It acts as a fundamental component for protein construction and is essential for chlorophyll production. Glycine also enhances the structural stability of plant cells and supports various metabolic activities. Due to its simple structure, glycine engages in numerous biochemical reactions, highlighting its versatility and significance in plants.
The metabolism of glycine encompasses the biochemical pathways responsible for its synthesis, utilization, and breakdown in plants. Employing advanced techniques and cutting-edge instrumentation, Creative Proteomics provides accurate and dependable data on glycine metabolism. Our service is designed to assist researchers and agricultural professionals in comprehending how glycine influences plant health, nutrient absorption, and growth.
Glycine Metabolism Analysis Service by Creative Proteomics
Detailed Glycine Metabolic Profiling
Our service focuses on analyzing glycine metabolism, including its synthesis, utilization, and breakdown pathways. By tracing glycine's path within plants, researchers can map its metabolic routes and identify key intermediates and end products.
Radiolabeled Glycine Tracing
We use radiolabeled glycine to monitor its metabolic fate, allowing researchers to observe its incorporation into various biochemical reactions within the plant.
Glycine Metabolism Enzyme Activity Assays
We perform enzyme activity assays to evaluate the function of enzymes involved in glycine metabolism. These assays help pinpoint critical regulatory points and understand the impact of enzymatic activity on glycine's metabolic pathways.
Techniques and Instrumentation for Glycine Metabolism Analysis
Liquid Chromatography (LC): Liquid Chromatography (LC) is used to separate and quantify glycine and its derivatives. By incorporating an Amide chromatography gradient, the system significantly improves the separation conditions for polar substances like amino acids.
Mass Spectrometry (MS): Advanced mass spectrometry techniques allow for the precise quantification of glycine and its metabolites, offering high-resolution insights into the metabolic flux. It enables the quantification of low-concentration and complex samples with unparalleled accuracy.
LC-MS/MS Method Establishment and Optimization: We establish precise quantification methods using reference standards to ensure accurate determination of glycine and related compounds. Multiple Reaction Monitoring (MRM)/Selected Reaction Monitoring (SRM) enhances the analysis and quantification of amino acids and peptides.
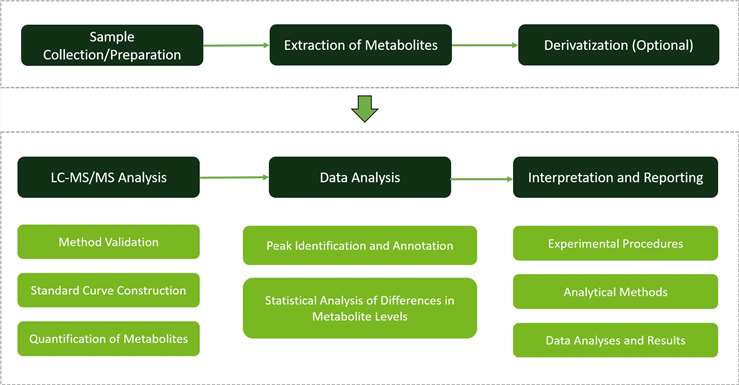
Why Choose Us?
- Comprehensive Insights: We cover all common plant sample types (roots, stems, leaves, flowers, fruits, etc.), with large identification numbers of metabolites, ensuring comprehensive metabolite profiling.
- Advanced Technology: We utilize the ultra-high-resolution Thermo Q-Exactive mass spectrometer, paired with Vanquish H UHPLC, for LC-MS/MS analysis of plant samples. This combination ensures that our analyses are both highly sensitive and precise.
- Customized Solutions: We tailor our analysis to meet the specific needs of each client, designing experiments and reports that address unique research questions, thus providing flexible and personalized solutions for diverse applications.
- Dual Quality Control: We implement strict quality control procedures and utilize multiple internal standards (including isotopic standards) to ensure that our data is reliable and accurate.
- Accurate Database Search: We use Progenesis QI software (version 3.0), a commercial mainstream database search software recognized by high-impact journals, ensuring efficient and accurate library searches.
- Smart Cloud Platform: Our service includes access to a comprehensive plant metabolomics cloud platform, which facilitates bioinformatics analysis and the creation of high-quality publication-ready images.
Applications of Glycine Metabolism Analysis
Pharmacology
- Drug Development: Insights into glycine metabolism can facilitate the development of new drugs that target specific metabolic pathways. This is crucial for creating medications that can modulate metabolic functions, particularly for conditions where metabolism plays a key role, such as liver diseases.
- Pharmacokinetics: Understanding glycine metabolism can help in predicting and improving the pharmacokinetics of drugs, as glycine conjugation is a common pathway for drug metabolism and detoxification.
Biochemical Research
- Enzyme Function Studies: Investigating the enzymes involved in glycine metabolism, such as glycine decarboxylase and serine hydroxymethyltransferase, can reveal important details about their function and regulation. This knowledge is essential for understanding broader biochemical processes.
- Pathway Elucidation: Analyzing the metabolic pathways of glycine contributes to a deeper understanding of amino acid metabolism and its integration with other metabolic networks, such as the folate cycle and the urea cycle.
Agriculture and Animal Husbandry
- Nutritional Optimization: Glycine is crucial for the growth and development of livestock. Analyzing and optimizing glycine metabolism can enhance feed formulations and improve animal health and productivity.
- Stress Response in Plants and Animals: Glycine metabolism analysis helps understand how plants and animals respond to stress. This knowledge can be used to develop more resilient crops and healthier livestock.
Food and Beverage Industry
- Nutritional Content Optimization: Analyzing glycine metabolism can help in formulating food products that are rich in glycine or enhance its bioavailability, promoting better health outcomes for consumers.
- Flavor Enhancement: Glycine is known to influence the taste of foods. Understanding its metabolism can assist in developing food products with improved flavor profiles.
Biotechnology
- Bioengineering: Glycine metabolism analysis can be used in bioengineering to produce microorganisms or plants with enhanced properties, such as increased growth rates or improved nutrient profiles.
- Fermentation Processes: In fermentation industries, optimizing glycine metabolism can lead to more efficient production processes and higher yields of desired products.
Sample Requirements for Glycine Metabolism Assay
| Sample Type |
Sample Volume |
Notes |
| Blood (Serum/Plasma) |
0.5 - 2 mL |
Collect in a fasting state if possible. Use EDTA or heparin tubes. |
| Urine |
10 - 50 mL |
First morning urine sample preferred. Collect in a sterile container. |
| Tissue Samples |
50 - 100 mg |
Fresh or frozen tissue. Ensure rapid freezing post-collection to -80°C. |
| Cell Culture |
1 - 5 x 10^6 cells |
Harvest cells and wash with PBS. Lyse cells for analysis. |
| Cerebrospinal Fluid (CSF) |
0.2 - 1 mL |
Collect via lumbar puncture, ensure sterility. |
| Saliva |
1 - 2 mL |
Collect unstimulated saliva in sterile tubes. |
| Feces |
5 - 10 g |
Collect in a sterile container. Ensure immediate processing or freezing. |
| Plant Leaves |
100 - 500 mg |
Collect fresh leaves, freeze immediately in liquid nitrogen. |
| Plant Roots |
100 - 500 mg |
Collect fresh roots, clean thoroughly, and freeze immediately. |
| Plant Seeds |
50 - 200 mg |
Collect mature seeds, store in a cool, dry place, or freeze. |
| Plant Stems |
100 - 500 mg |
Collect fresh stems, freeze immediately in liquid nitrogen. |
Each experimental treatment should have more than 6 biological replicates to ensure robust statistical analysis and reliable interpretation of results.
For other sample types not listed above, such as flowers or whole plants, please consult our technical support or sales team for specific requirements and recommendations.
Case. Glycine-Induced Phosphorylation Plays a Pivotal Role in Energy Metabolism in Roots and Amino Acid Metabolism in Leaves of Tea Plant
Background:
Despite extensive research on ammonium and nitrate nitrogen, organic nitrogen's contributions remain less understood.
Glycine, due to its high solubility and low molecular weight, serves as an ideal model for studying organic nitrogen uptake.
This study investigates the regulatory role of glycine-induced phosphorylation in tea plants, focusing on its effects on energy metabolism in roots and amino acid metabolism in leaves.
Samples:
Two-year-old clonal tea seedlings (Camellia sinensis cv. 'Lianshan 10') were hydroponically grown under controlled greenhouse conditions.
After two weeks in a standard nutrient solution, seedlings were divided into two groups: a control group with nitrogen-deficient solution and an experimental group with 1.00 mM glycine.
Samples from leaves and roots were collected, frozen, and stored at -80°C.
Technical methods procedure:
Photosynthetic rate and chlorophyll content were measured using a portable photosynthesis system and a chlorophyll meter, respectively. Enzyme activities of several key enzymes were determined using commercially available kits.
Total nitrogen content was determined by the Kjeldahl method. Amino acid contents were analyzed using HPLC following sample hydrolysis, centrifugation, and derivatization with phenyl isothiocyanate.
Metabolites were extracted from freeze-dried samples, followed by UPLC-ESI-MS/MS analysis. Conditions included an Agilent SB-C18 column and a mobile phase gradient. Metabolites were identified and quantified using the Q TRAP mass spectrometry system.
Phosphoproteins were extracted, digested with trypsin, and enriched using immobilized metal affinity chromatography. LC-MS/MS analysis was conducted using an EASY-nLC 1000 UPLC system and Orbitrap Fusion Lumos mass spectrometer. Data were processed with the Maxquant search engine to identify phosphorylated peptides and proteins.
Results
Glycine inhibited root growth but enhanced leaf greenness. Chlorophyll content and photosynthetic rate were higher with glycine. Nitrogen content increased in both roots and leaves, indicating glycine provided nitrogen nutrition and altered nitrogen distribution.
Glycine enhanced amino acid metabolism in roots and altered amino acid distribution in leaves, increasing some amino acids while decreasing others.
Glycine affected the activities of key enzymes, increasing GS and decreasing Fd-GOGAT in leaves. Variations in SuSy, GAPDH, PGM, and other enzymes were observed, indicating glycine's impact on enzyme activity.
Glycine altered metabolite levels, increasing glycolysis-related metabolites in roots but decreasing them in leaves. TCA cycle metabolites varied, with α-ketoglutaric acid lower in glycine-treated leaves, suggesting its role in amino acid metabolism.
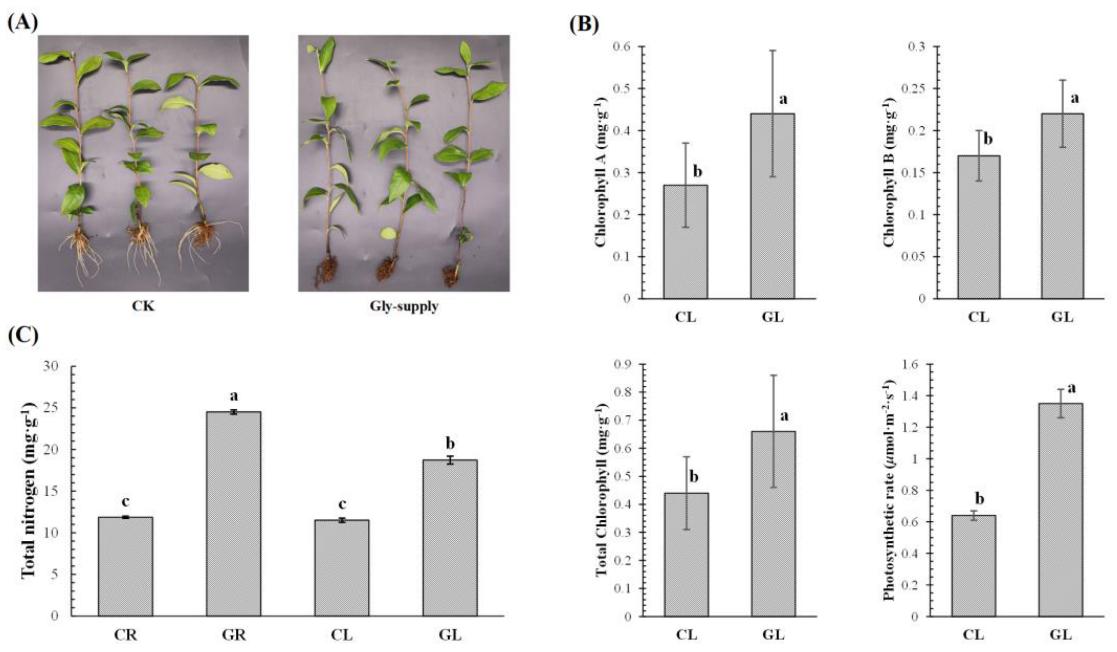 Fig 1. (A) Phenotypes of tea plants. (B) The chlorophyll contents and net photosynthetic rates of tea leaves. (C) The nitrogen contents of tea roots and leaves. Different lowercase letters indicated significant differences according to one-way ANOVA (p < 0.05).
Fig 1. (A) Phenotypes of tea plants. (B) The chlorophyll contents and net photosynthetic rates of tea leaves. (C) The nitrogen contents of tea roots and leaves. Different lowercase letters indicated significant differences according to one-way ANOVA (p < 0.05).
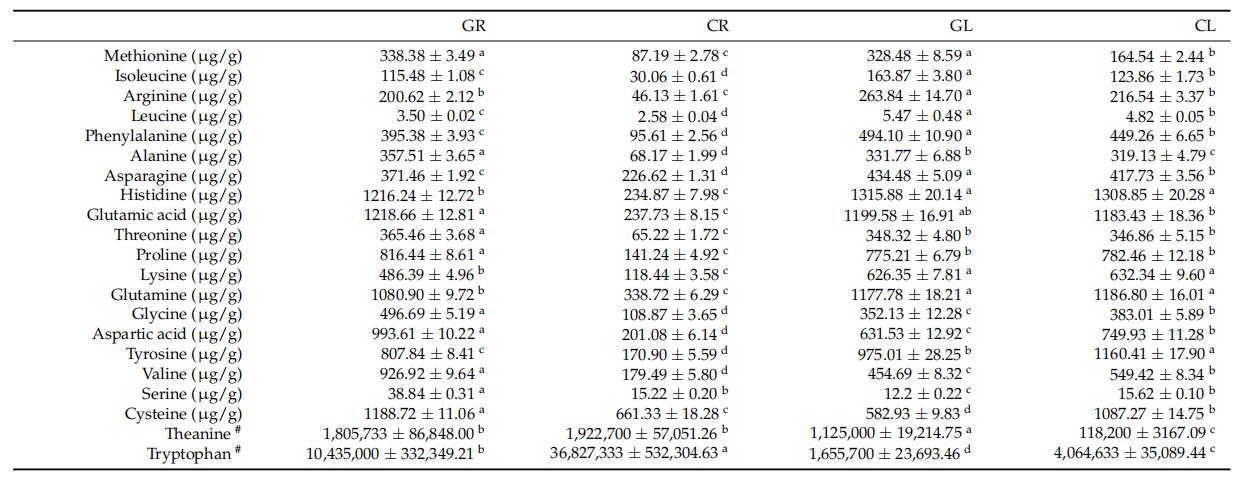 Fig 2. Contents of amino acids in tea roots and leaves. Contents of amino acids were presented as mean ± standard deviation. Different lowercase letters indicated significant differences according to the Duncan test at p < 0.05. # The data came from the results of metabolomics (relative value).
Fig 2. Contents of amino acids in tea roots and leaves. Contents of amino acids were presented as mean ± standard deviation. Different lowercase letters indicated significant differences according to the Duncan test at p < 0.05. # The data came from the results of metabolomics (relative value).
Reference
- Li, Y. (2023). " Glycine-Induced Phosphorylation Plays a Pivotal Role in Energy Metabolism in Roots and Amino Acid Metabolism in Leaves of Tea Plant." Foods 12(2), 334.



 Fig 1. (A) Phenotypes of tea plants. (B) The chlorophyll contents and net photosynthetic rates of tea leaves. (C) The nitrogen contents of tea roots and leaves. Different lowercase letters indicated significant differences according to one-way ANOVA (p < 0.05).
Fig 1. (A) Phenotypes of tea plants. (B) The chlorophyll contents and net photosynthetic rates of tea leaves. (C) The nitrogen contents of tea roots and leaves. Different lowercase letters indicated significant differences according to one-way ANOVA (p < 0.05). Fig 2. Contents of amino acids in tea roots and leaves. Contents of amino acids were presented as mean ± standard deviation. Different lowercase letters indicated significant differences according to the Duncan test at p < 0.05. # The data came from the results of metabolomics (relative value).
Fig 2. Contents of amino acids in tea roots and leaves. Contents of amino acids were presented as mean ± standard deviation. Different lowercase letters indicated significant differences according to the Duncan test at p < 0.05. # The data came from the results of metabolomics (relative value).

