Why Analyze Pachypodol?
Pachypodol is an O-methylated flavonoid that is a natural constituent of a wide variety of plants and herbs. This compound is of increasing interest to the scientific community due to its many implications in promoting human health. Pachypodol has a wide range of biological activities including antioxidant, anti-inflammatory, anti-microbial and even anti-carcinogenic.
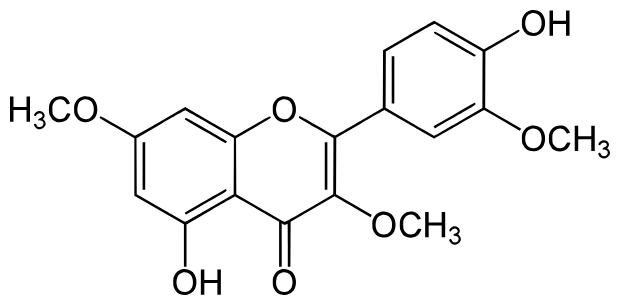 Pachypodol
Pachypodol
As a natural compound that plays an important role in biochemical reactions, it is crucial to understand the specifics of pachypodol concentration, distribution and behavior in various biological systems.
By analyzing the properties of pachypodol, we can accurately decipher its potency, detect its presence in various matrices, and assess its stability in these matrices, thus furthering its potential industrial or medical applications.
In addition, pachypodol analysis helps to comprehensively study its pharmacokinetics and toxicokinetics, two important parameters that have a significant impact on the safety and efficacy of therapeutic interventions. It helps to answer questions related to the absorption, distribution, metabolism and excretion (ADME) of pachypodol in living organisms.
Creative Proteomics offers state-of-the-art Pachypodol Analysis Services that cater to various research needs
Specific Pachypodol Analysis Offered by Creative Proteomics
Our services encompass a broad spectrum of project directions, designed to meet the diverse needs of our clients. These include:
- Qualitative and Quantitative Analysis: Utilizing advanced chromatography and mass spectrometry techniques, we offer precise qualitative and quantitative analysis of pachypodol in various matrices.
- Purity Analysis and Standardization: We provide comprehensive services for determining the purity of pachypodol and standardizing herbal extracts and supplements.
- Metabolite Profiling: Our expertise extends to the profiling of pachypodol metabolites, which is crucial for understanding its pharmacokinetics and pharmacodynamics.
- Pharmacological Activity Screening: Leveraging our state-of-the-art analytical platforms, we conduct screening for the pharmacological activities of pachypodol, aiding in the discovery of potential therapeutic applications.
Pachypodol Analysis Techniques
Liquid Chromatography-Mass Spectrometry (LC-MS/MS)
This technique synergistically merges the separation prowess of high-performance liquid chromatography (HPLC) with the detailed mass analysis afforded by mass spectrometry (MS). The implementation of tandem mass spectrometry (MS/MS) augments both sensitivity and specificity, facilitating the discernment and quantitation of pachypodol amidst intricate biological matrices. LC-MS/MS excels in the determination of molecular weight and structural intricacies of pachypodol, thereby advancing our understanding of its pharmacokinetic profiles and bioavailability.
Ultra-High Performance Liquid Chromatography (UHPLC)
UHPLC, an advancement over HPLC, offers faster analysis and higher resolution, crucial for separating pachypodol from similar compounds and impurities. Operating at increased pressures, UHPLC uses smaller particle size columns, enhancing separation efficiency and characterizing pachypodol more precisely. Coupled with MS, UHPLC accurately quantifies pachypodol.
Gas Chromatography-Mass Spectrometry (GC-MS)
For volatile and semi-volatile pachypodol derivatives, GC-MS is preferred. It integrates gas chromatography's efficient separation with mass spectrometry's precise analysis. Particularly suited for essential oils and aromatic plant extracts, GC-MS identifies and quantifies trace pachypodol amounts, shedding light on its botanical chemical ecology and therapeutic potential.
Nuclear Magnetic Resonance (NMR) Spectroscopy
Nuclear Magnetic Resonance (NMR) spectroscopy offers critical structural insights into pachypodol, elucidating its molecular framework essential for understanding biological activity. By revealing atom arrangements, NMR provides clues to pachypodol's binding affinities and interactions with biological targets. This non-destructive technique is pivotal for confirming pachypodol purity and identity in research and development.
 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service
Sample Requirements for Pachypodol Assay
| Sample Type |
Recommended Sample Volume |
| Plant Extracts |
100-500 mg |
| Essential Oils |
10-50 µL |
| Herbal Supplements |
50-200 mg |
| Biological Matrices (e.g., serum/plasma) |
100-500 µL |
| Crude Plant Material |
500-1000 mg |
| Aqueous Extracts |
1-5 mL |
| Solid Herbal Products |
100-500 mg |
| Cell Culture Supernatants |
0.5-1 mL |
| Tissue Homogenates |
50-200 mg |
| Soil Samples |
1-5 g |
Deliverables of Pachypodol Analysis
- Detailed report of the methodologies applied, including conditions and parameters
- Quantitative data on pachypodol content in the investigated samples
- Comprehensive analysis of pachypodol metabolic profile
- Insights into the biological effects of pachypodol
- Assessment of pachypodol's potential toxicity
- Expert interpretation and recommendations for further research or applications
Applications of Pachypodol Analysis
Drug Discovery and Development
Pachypodol analysis plays a pivotal role in drug discovery efforts, serving as a valuable tool for identifying potential lead compounds with therapeutic efficacy. By elucidating the structural characteristics and pharmacological activities of pachypodol and its derivatives, we can explore novel drug candidates for the treatment of various diseases, including cancer, inflammation, and neurodegenerative disorders.
Quality Control in Herbal Products
As pachypodol is commonly found in medicinal plants and herbal supplements, its analysis is crucial for ensuring the quality, authenticity, and efficacy of herbal products. By quantifying the content of pachypodol and monitoring for adulteration or contamination, quality control measures can be implemented to safeguard consumer health and maintain product integrity.
Pharmacokinetic Studies
Pachypodol analysis facilitates pharmacokinetic studies aimed at understanding the absorption, distribution, metabolism, and excretion (ADME) properties of this bioactive compound. By tracking the concentration-time profiles of pachypodol in biological matrices such as plasma or urine, we can gain insights into its bioavailability, metabolic fate, and potential interactions with other drugs.
Pharmacological Research
The analysis of pachypodol enables we to investigate its pharmacological effects and mechanisms of action in various biological systems. From antioxidant and anti-inflammatory activities to anticancer and neuroprotective properties, pachypodol exhibits a wide range of pharmacological activities that can be explored for therapeutic interventions in diverse disease conditions.
Environmental Monitoring
Pachypodol analysis can also find applications in environmental monitoring studies, particularly in assessing its presence and distribution in natural ecosystems. By analyzing pachypodol levels in soil, water, or plant samples, we can evaluate its ecological significance, potential ecological risks, and environmental impact.
Case. Comprehensive Analysis of Methoxylated Flavones: Pharmacokinetic Insights and Analytical Method Development
Background
Methoxylated flavones, such as kumatakenin, pachypodol, and retusin, exhibit potential pharmacological properties, necessitating accurate analytical methods for their quantification in biological samples. Understanding their pharmacokinetics is crucial for drug development.
Sample
Rat plasma samples were utilized for pharmacokinetic studies. Methoxylated flavones were extracted using liquid-liquid extraction with acidified ethyl acetate, ensuring efficient analyte recovery and matrix effect minimization.
Technical Platform and Procedure
The analytical method employed ultra-high-performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) in the positive ion electrospray ionization mode for sensitive and selective detection of kumatakenin, pachypodol, and retusin. Chromatographic separation was achieved using a reverse-phase C18e column, with optimized mobile phase composition (acetonitrile:water with 0.1% formic acid) to enhance peak shape and resolution. Sample preparation involved liquid-liquid extraction (LLE) with acidified ethyl acetate, selected for its efficiency in extracting the target analytes from rat plasma while minimizing interference.
Results
The developed UHPLC-MS/MS method demonstrated excellent performance characteristics, including high selectivity, linearity, sensitivity, and precision, for the quantification of kumatakenin, pachypodol, and retusin in rat plasma. Stability studies revealed satisfactory short-term stability under various conditions but indicated compromised long-term stability, particularly with repeated freeze-thaw cycles and extended storage durations. Pharmacokinetic evaluation in rats revealed significant differences in key parameters, such as area under the curve (AUC), clearance (Cl), half-life (t1/2), and mean residence time (MRT), between pachypodol and retusin administration groups, suggesting distinct metabolic profiles influenced by hydrophobicity.
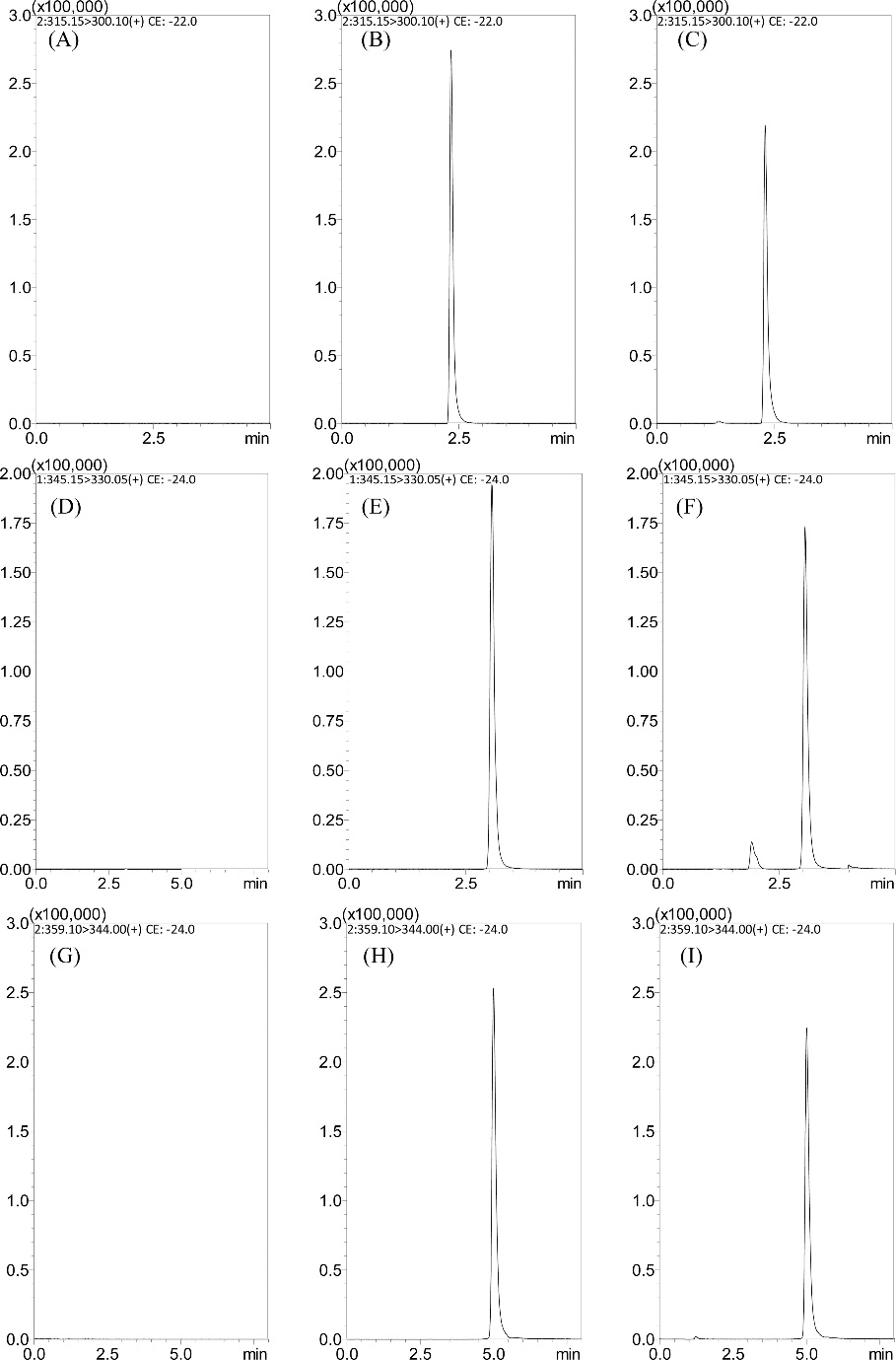 Representative SRM chromatograms of (A) a blank plasma sample; (B) a blank plasma sample spiked with kumatakenin (100 ng/mL); (C) a plasma sample at 30 min after administration of kumatakenin (3 mg/kg, i.v.); (D) a blank plasma sample; (E) a blank plasma sample spiked with pachypodol (100 ng/mL); (F) a plasma sample at 30 min after administration of pachypodol (3 mg/kg, i.v.); (G) a blank plasma sample; (H) a blank plasma sample spiked with retusin (100 ng/mL); and (I) a plasma sample at 30 min after administration of retusin (3 mg/kg, i.v.).
Representative SRM chromatograms of (A) a blank plasma sample; (B) a blank plasma sample spiked with kumatakenin (100 ng/mL); (C) a plasma sample at 30 min after administration of kumatakenin (3 mg/kg, i.v.); (D) a blank plasma sample; (E) a blank plasma sample spiked with pachypodol (100 ng/mL); (F) a plasma sample at 30 min after administration of pachypodol (3 mg/kg, i.v.); (G) a blank plasma sample; (H) a blank plasma sample spiked with retusin (100 ng/mL); and (I) a plasma sample at 30 min after administration of retusin (3 mg/kg, i.v.).
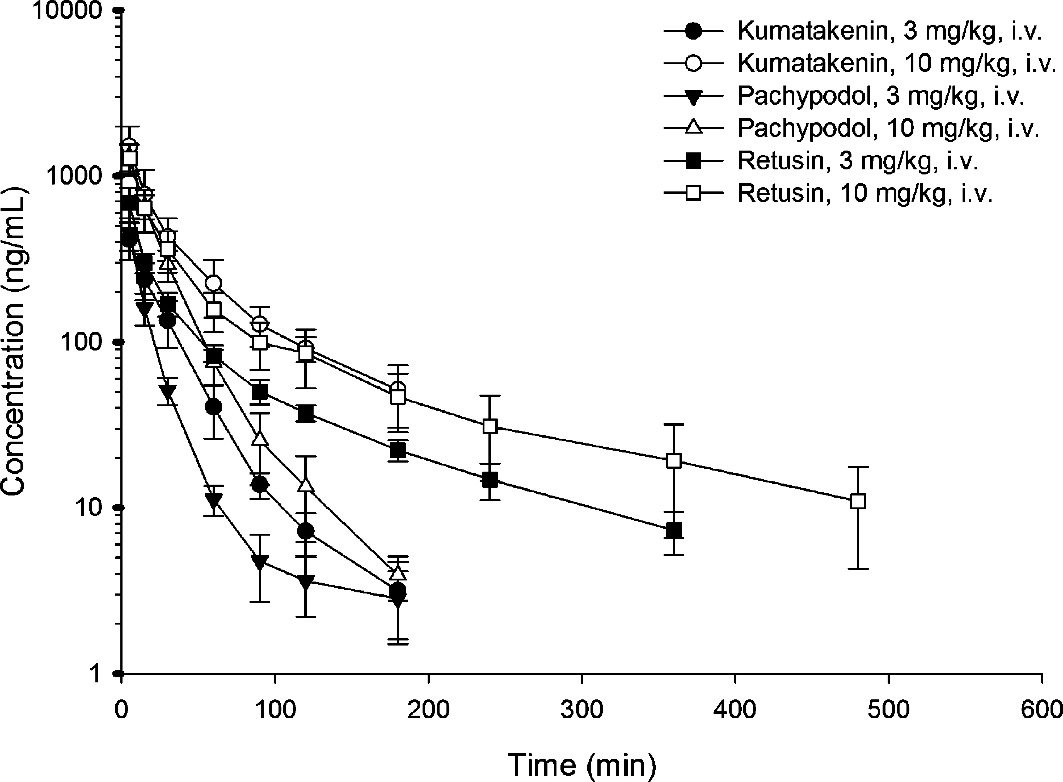 Drug concentration versus time profiles of kumatakenin, pachypodol, and retusin in rat plasma after drug administration (3 mg/kg, i.v. and 10 mg/kg)
Drug concentration versus time profiles of kumatakenin, pachypodol, and retusin in rat plasma after drug administration (3 mg/kg, i.v. and 10 mg/kg)
Reference
- Huang, Jing-Ting, et al. "Structural pharmacokinetics of polymethoxylated flavones in rat plasma using HPLC-MS/MS." Journal of agricultural and food chemistry 65.11 (2017): 2406-2413.


 Pachypodol
Pachypodol Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service Representative SRM chromatograms of (A) a blank plasma sample; (B) a blank plasma sample spiked with kumatakenin (100 ng/mL); (C) a plasma sample at 30 min after administration of kumatakenin (3 mg/kg, i.v.); (D) a blank plasma sample; (E) a blank plasma sample spiked with pachypodol (100 ng/mL); (F) a plasma sample at 30 min after administration of pachypodol (3 mg/kg, i.v.); (G) a blank plasma sample; (H) a blank plasma sample spiked with retusin (100 ng/mL); and (I) a plasma sample at 30 min after administration of retusin (3 mg/kg, i.v.).
Representative SRM chromatograms of (A) a blank plasma sample; (B) a blank plasma sample spiked with kumatakenin (100 ng/mL); (C) a plasma sample at 30 min after administration of kumatakenin (3 mg/kg, i.v.); (D) a blank plasma sample; (E) a blank plasma sample spiked with pachypodol (100 ng/mL); (F) a plasma sample at 30 min after administration of pachypodol (3 mg/kg, i.v.); (G) a blank plasma sample; (H) a blank plasma sample spiked with retusin (100 ng/mL); and (I) a plasma sample at 30 min after administration of retusin (3 mg/kg, i.v.). Drug concentration versus time profiles of kumatakenin, pachypodol, and retusin in rat plasma after drug administration (3 mg/kg, i.v. and 10 mg/kg)
Drug concentration versus time profiles of kumatakenin, pachypodol, and retusin in rat plasma after drug administration (3 mg/kg, i.v. and 10 mg/kg)

