What is Naringenin?
In the realm of phytochemistry, the pursuit of understanding the intricate properties of plant-derived compounds has led researchers to uncover a plethora of bioactive molecules with promising therapeutic potential. Among these compounds, naringenin stands out as a potent flavanone, abundant in various citrus fruits such as grapefruits and oranges. Its chemical structure, characterized by a flavanone backbone with hydroxyl groups at specific positions, imbues naringenin with remarkable antioxidant, anti-inflammatory, and anticancer properties.
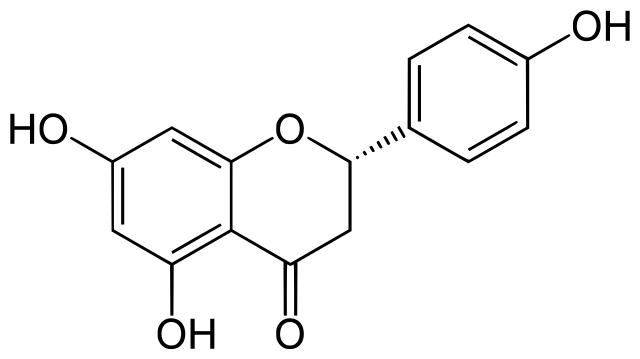 Naringenin
Naringenin
Naringenin's multifaceted bioactivity makes it a subject of intense research interest. Studies have shown its potential in modulating various molecular pathways implicated in inflammation, oxidative stress, and carcinogenesis. However, to fully harness its therapeutic potential, researchers must elucidate naringenin's pharmacokinetic profile, including its absorption, distribution, metabolism, and excretion (ADME) characteristics. Accurate analysis of naringenin in biological samples is indispensable for unraveling these complex interactions and determining optimal dosage regimens for therapeutic applications.
The widespread incorporation of naringenin into nutraceuticals, dietary supplements, and functional foods underscores the importance of rigorous quality control measures. Ensuring the purity, potency, and stability of naringenin-containing products is essential for safeguarding consumer health and maintaining regulatory compliance. Analytical techniques capable of detecting naringenin at trace levels in complex matrices are indispensable for verifying product authenticity and consistency.
Creative Proteomics offers a comprehensive naringenin analysis service to advance research on this promising bioactive compound.
Naringenin Analysis Offered by Creative Proteomics
- Naringenin Identification: Utilizing advanced analytical techniques to accurately identify and confirm the presence of naringenin in various samples.
- Quantitative Analysis: Precise quantification of naringenin content in samples using validated methodologies such as HPLC, LC-MS, or GC-MS.
- Structural Characterization: Elucidation of the chemical structure of naringenin and its derivatives through spectroscopic analysis (NMR, MS/MS).
- Purity Assessment: Assessment of the purity of naringenin preparations to ensure high quality and consistency.
- Stability Studies: Investigation of the stability of naringenin under different storage conditions, providing insights for optimal handling and storage.
- Pharmacokinetic Studies: Evaluation of the absorption, distribution, metabolism, and excretion (ADME) properties of naringenin in biological systems.
- Bioavailability Assessment: Determination of the bioavailability of naringenin formulations to assess their efficacy and potential for therapeutic applications.
- Toxicity Evaluation: Assessment of the safety profile and potential toxic effects of naringenin using in vitro and in vivo models.
Technological Platforms for Naringenin Analysis
Creative Proteomics harnesses the power of advanced mass spectrometry (MS)-based platforms for the analysis of naringenin, ensuring unparalleled precision and sensitivity. Our state-of-the-art instruments include:
- Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS): The Agilent 6495B Triple Quadrupole LC/MS system offers high sensitivity and accuracy, ideal for quantifying naringenin and its metabolites.
- High-Resolution Mass Spectrometry (HRMS): The Thermo Fisher Scientific Orbitrap Exploris 480 provides exceptional resolution and mass accuracy, facilitating comprehensive metabolite profiling.
- Ultra-Performance Liquid Chromatography (UPLC): Coupled with MS, systems like the Waters Acquity UPLC H-Class with Xevo G2-XS QTof enable rapid and precise analysis of naringenin in complex biological matrices.
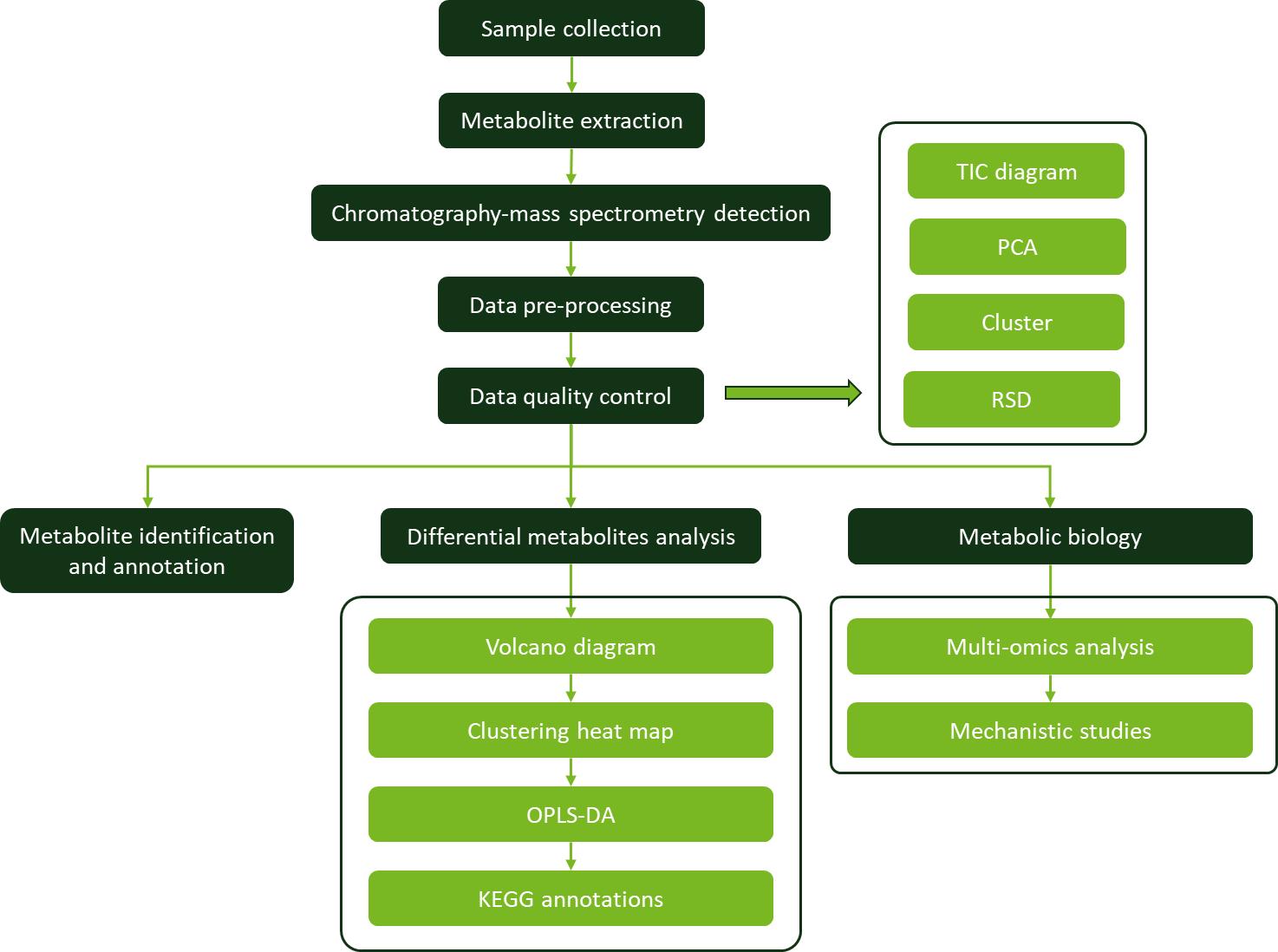 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service
Sample Requirements for Naringenin Assay
| Sample Type |
Sample Volume |
| Biological tissues |
10-100 mg (e.g., liver, kidney) |
| Microbial cultures |
1-10 mL |
| Agricultural products |
5-20 g |
| Herbal supplements |
50-200 mg |
| Dietary supplements |
50-200 mg |
| Pharmaceuticals |
10-50 mg |
| Environmental samples |
Variable (depending on matrix) |
| Beverage products |
10-50 mL (e.g., fruit juice, tea) |
| Soil and sediment samples |
1-10 g |
Deliverables of Naringenin Analysis
Our delivery includes a detailed naringenin analysis report that demonstrates the comprehensive data that we extracted, thoroughly analyzed and accurately interpreted. This data includes sample preparation information, details of adjustments made during the analysis, and the final results of the spectra.
In addition, we provide raw data with detailed explanations of the techniques used and calculations performed. You also get a comprehensive overview of the peaks we determined, the naringenin components and their relative abundance. All these valuable results will endeavor to meet your expectations for results, facilitate your research progress and support your risk assessment or product development efforts.
Applications of Naringenin Analysis
Drug Discovery and Development: Naringenin serves as a valuable lead compound in the discovery of novel pharmaceutical agents. Analyzing its pharmacokinetics and bioavailability aids in the development of efficacious drugs targeting inflammation, oxidative stress, and cancer.
Nutraceutical and Dietary Supplement Formulation: The incorporation of naringenin into nutraceuticals and dietary supplements is driven by its antioxidant and anti-inflammatory properties. Analyzing naringenin content ensures product quality, potency, and compliance with regulatory standards.
Pharmacokinetic Studies: Understanding the absorption, distribution, metabolism, and excretion (ADME) of naringenin is essential for assessing its pharmacological effects and optimizing dosage regimens. Naringenin analysis enables the characterization of its pharmacokinetic profile in biological systems.
Biomarker Discovery: Naringenin metabolites may serve as potential biomarkers for assessing dietary intake and metabolic processes. Analytical techniques facilitate the identification and quantification of naringenin-derived metabolites, offering insights into its physiological effects.
Functional Food Development: Incorporating naringenin-rich ingredients into functional foods enhances their nutritional value and health-promoting properties. Analyzing naringenin levels ensures the efficacy and consistency of these products, catering to consumer demand for natural health solutions.
Health Research and Disease Prevention: Research on naringenin's health benefits extends to disease prevention and management, including cardiovascular diseases, neurodegenerative disorders, and metabolic syndromes. Naringenin analysis contributes to elucidating its mechanisms of action and potential therapeutic applications.
Case. Pharmacokinetic comparisons of naringenin and naringenin-nicotinamide cocrystal in rats by LC-MS/MS
Background
Naringenin (NAR), a dihydroflavonoid abundant in citrus fruits, exhibits promising pharmacological activities but suffers from poor solubility and low bioavailability. To address this limitation, the present study focuses on exploring the potential of a naringenin-nicotinamide (NAR-NCT) cocrystal to enhance NAR's bioavailability.
Sample
The study utilized NAR and nicotinamide obtained from reputable suppliers. Rat plasma samples were collected for pharmacokinetic analysis following oral administration of NAR, a physical mixture of NAR and nicotinamide (NAR + NCT), and NAR-NCT.
Technical Methods
Preparation and characterization of NAR-NCT cocrystal: NAR and nicotinamide were mixed at a molar ratio of 1 : 2 in ethyl acetate, followed by solvent evaporation. Characterization involved differential scanning calorimetry (DSC), X-ray powder diffraction (XRPD), and infrared spectroscopy (IR).
Instrumentation: LC-MS/MS analysis was performed using a Shimadzu UFLC-20AD XR system coupled with an AB SCIEX Qtrap 5500 mass spectrometer.
Method Validation: Specificity, calibration curve construction, precision, accuracy, extraction recovery, matrix effect, and stability of NAR-NCT in rat plasma were evaluated following US FDA guidelines.
Pharmacokinetic Study: Eighteen Sprague-Dawley rats were orally administered with NAR, NAR + NCT, and NAR-NCT. Serial blood samples were collected, and plasma concentrations of NAR were determined using LC-MS/MS. Pharmacokinetic parameters were calculated using noncompartmental analysis.
Results
Characterization of NAR-NCT cocrystal: DSC, XRPD, and IR spectra confirmed the formation of a new NAR-NCT cocrystal.
Stability of NAR-NCT: NAR-NCT remained stable under various conditions, as evidenced by consistent XRPD patterns.
Optimization of Plasma Sample Preparation: Ethyl acetate demonstrated superior extraction efficiency for NAR from plasma samples.
Method Validation: The developed method exhibited good linearity, precision, accuracy, and stability, meeting validation criteria.
Pharmacokinetics and Bioavailability: Compared to NAR alone, both NAR + NCT and NAR-NCT formulations showed improved pharmacokinetic profiles, with NAR-NCT demonstrating significantly enhanced bioavailability (175.09% compared to NAR alone) attributed to faster absorption and slower elimination.
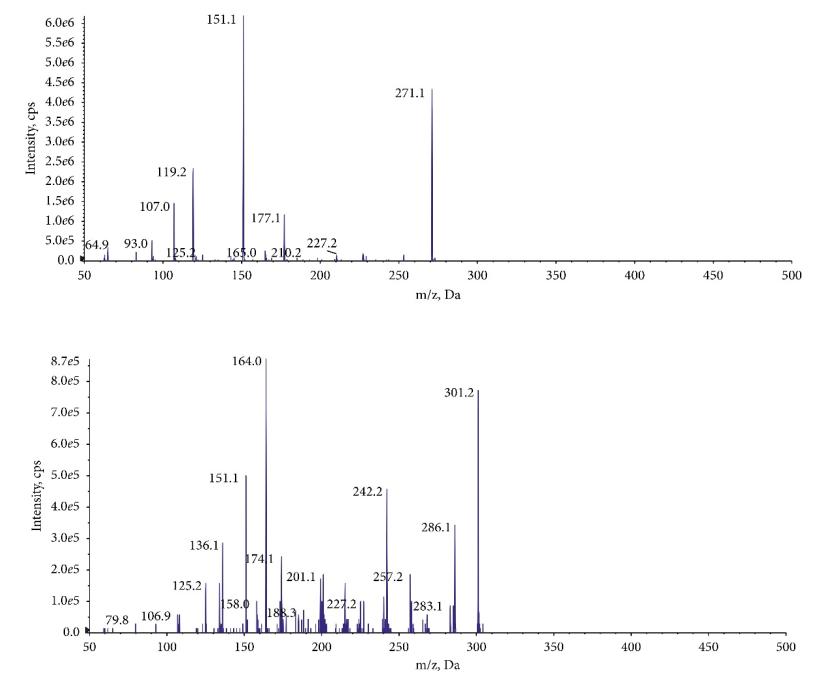 Mass spectrometry of the product ion: (a) NAR and (b) IS.
Mass spectrometry of the product ion: (a) NAR and (b) IS.
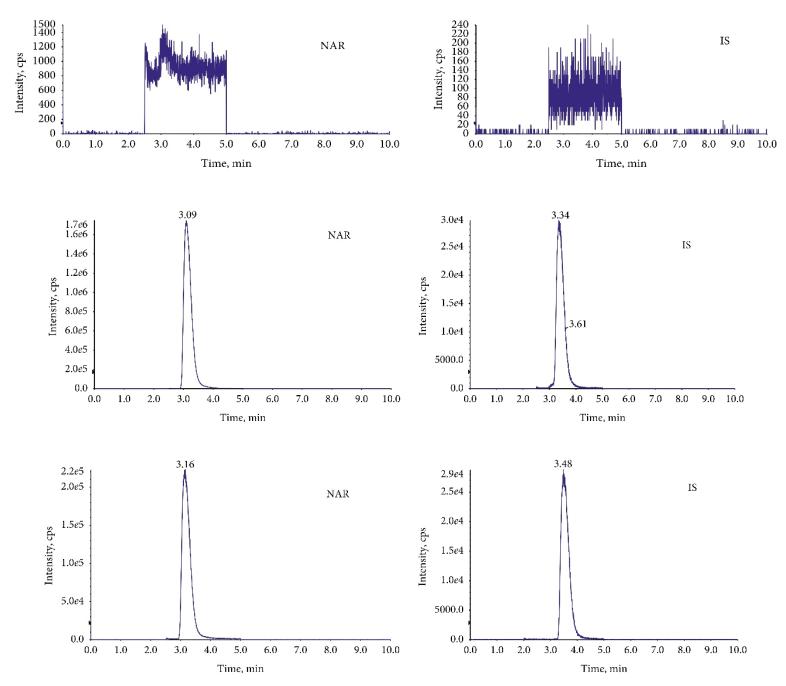 The chromatograms of MRM: (a) blank plasma, (b) plasma mixed with NAR, and (c) plasma after oral administration of NAR.
The chromatograms of MRM: (a) blank plasma, (b) plasma mixed with NAR, and (c) plasma after oral administration of NAR.
Reference
- Xu, Dan, et al. "Pharmacokinetic comparisons of naringenin and naringenin-nicotinamide cocrystal in rats by LC-MS/MS." Journal of analytical methods in chemistry 2020 (2020).


 Naringenin
Naringenin Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service Mass spectrometry of the product ion: (a) NAR and (b) IS.
Mass spectrometry of the product ion: (a) NAR and (b) IS. The chromatograms of MRM: (a) blank plasma, (b) plasma mixed with NAR, and (c) plasma after oral administration of NAR.
The chromatograms of MRM: (a) blank plasma, (b) plasma mixed with NAR, and (c) plasma after oral administration of NAR.

