What is Cyanidin?
Cyanidin is a natural organic compound belonging to the class of pigments known as anthocyanins, which are a type of flavonoid. These compounds are responsible for the vibrant red, purple, and blue colors found in many fruits, vegetables, and flowers. Chemically, cyanidin is known for its antioxidant properties, helping to protect cells against damage from free radicals.
Cyanidin and other anthocyanins are not only important for the coloration of plants but also contribute to various health benefits when consumed in the human diet. Studies suggest that these compounds may help reduce the risk of cardiovascular disease, support brain health, and contribute to anti-inflammatory and anti-carcinogenic activities. They are commonly found in foods like berries (such as blueberries, raspberries, and blackberries), red grapes, and cherries, as well as in red cabbage and red onions.
Cyanidin Analysis Offered by Creative Proteomics
Creative Proteomics offers a comprehensive suite of services tailored for cyanidin analysis. These services are designed to meet the needs of various stakeholders in the food science, pharmacology, and nutraceutical industries. Our offerings include:
- Quantitative and Qualitative Analysis: Utilizing high-throughput technologies to measure cyanidin content and profile its derivatives in complex biological matrices.
- Metabolite Profiling: Identifying and quantifying cyanidin metabolites to understand their biological impacts better.
- Bioavailability Studies: Assessing how cyanidin compounds are absorbed, metabolized, and excreted in the human body, crucial for dietary supplement development and nutritional research.
- Stability and Degradation Assessments: Evaluating how processing and storage conditions affect the stability of cyanidins in food products.
Technological Platforms for Cyanidin Analysis
High-Performance Liquid Chromatography (HPLC)
Our HPLC systems are outfitted with:
- Agilent 1260 Infinity II HPLC System: Featuring high-resolution chromatography and sensitive detection capabilities, allowing for precise separation and quantification of cyanidins.
- Waters Alliance HPLC System: Known for its robust performance and versatility in handling complex sample matrices, facilitating accurate analysis of cyanidin compounds.
Mass Spectrometry (MS)
We employ:
- Thermo Scientific Q Exactive Plus Orbitrap Mass Spectrometer: Offering high-resolution mass detection and tandem mass spectrometry (MS/MS) capabilities for accurate identification and structural elucidation of cyanidins and their metabolites.
- AB SCIEX Triple Quad 6500 LC-MS/MS System: Recognized for its sensitivity and speed in quantifying trace-level analytes, essential for precise measurement of cyanidin concentrations.
Nuclear Magnetic Resonance (NMR) Spectroscopy
Our NMR facility features:
- Bruker AVANCE III HD NMR Spectrometer: Equipped with ultra-high-field magnet and advanced probe technology, enabling detailed structural elucidation of cyanidin molecules and their interactions.
Ultraviolet-visible (UV-Vis) Spectrophotometry
We utilize:
- Shimadzu UV-2600 UV-Vis Spectrophotometer: Known for its accuracy and reliability in quantifying cyanidin content through UV absorbance measurements.
Integration with Bioinformatics Tools
We integrate sophisticated bioinformatics software, including:
- PeakView Software: Facilitating data processing and analysis for LC-MS/MS results, aiding in the identification and quantification of cyanidin compounds.
- MestreNova NMR Software: Enabling efficient processing and interpretation of NMR spectra for structural elucidation of cyanidins.
 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service
Sample Requirements for Cyanidin Assay
| Sample Type |
Sample Quantity |
| Fruit Extracts |
1-5 grams |
| Vegetable Extracts |
1-5 grams |
| Plant Tissues |
0.5-2 grams |
| Dietary Supplements |
50-200 milligrams |
| Serum/Plasma |
100-500 microliters |
| Urine |
1-5 milliliters |
| Food Products |
Dependent on product type |
Applications of Cyanidin Analysis
Food Science and Nutrition: Determining cyanidin levels helps assess nutritional quality and antioxidant capacity in foods, guiding processing methods and storage.
Pharmaceuticals and Herbal Medicine: Analyzing cyanidin in medicinal plants ensures standardized herbal remedies, aiding in consistent dosage and efficacy.
Plant Breeding and Genetics: Understanding cyanidin accumulation aids in breeding crops with enhanced nutritional profiles and disease resistance.
Biomedical Research: Investigating cyanidin's health effects supports potential therapeutic applications in preventing or treating diseases.
Environmental Monitoring: Monitoring cyanidin levels in plants offers insights into environmental stress responses and ecosystem health.
Quality Control in Food and Beverage Industry: Cyanidin analysis verifies the authenticity and purity of natural colorants, meeting regulatory standards and consumer expectations.
Case. Acylation of Cyanidin-3-glucoside (C3G) from Blueberry: Methods and Stability Analysis
Background
Anthocyanins, particularly Cyanidin-3-glucoside (C3G), are potent bioactive flavonoids found in fruits like blueberries. They possess attractive color properties and various health benefits due to their antioxidant and anti-inflammatory properties. However, their application as natural colorants is limited by factors such as instability and poor solubility in oils. Acylation has been proposed as a method to enhance the stability and lipotropic properties of anthocyanins, making them more suitable for food applications.
Sample
C3G was extracted from blueberry, containing 80% w/w C3G. Lauric acid was used as the acyl donor substrate, along with other reagents such as 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide, N-hydroxybenzotriazole, and solvents including DMF and TFA.
Technical Methods
1. Chemical Acylation Procedure: C3G was combined with acyl donor substrates in DMF solvent under argon gas protection. The reaction was stirred for 48 hours at 4°C, with samples collected every 12 hours for analysis by HPLC-MS.
2. Preparative High Performance Liquid Chromatography (PHPLC) Separation and Purification: The acylation product was purified using PHPLC with an X-Bridge C18 preparative column and ACN/Water + TFA solvent system.
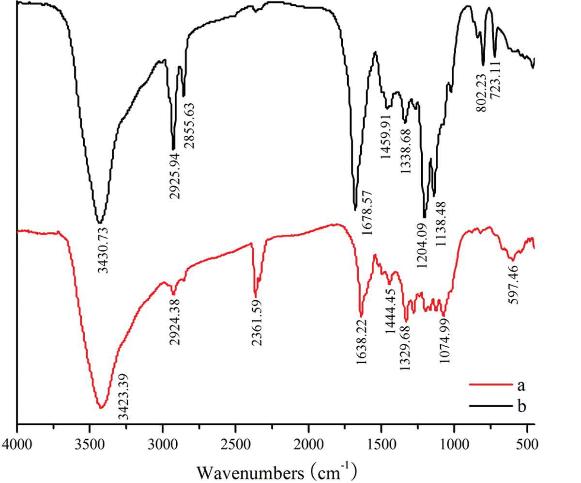
3. Fourier Transform Infrared (FTIR) Analysis: C3G and acylated C3G samples were analyzed using an FTIR spectrometer to characterize molecular structures.
4. Liquid Chromatography-Mass Spectrometry (LC-MS) Analysis: LC-MS was performed using an ICQ Deca ion trap mass spectrometer coupled with HPLC to analyze the acylation products.
5. Stability Analysis of Acylated C3G: The stability of acylated C3G was evaluated under different conditions, including temperature and light exposure, as well as in the presence of various additives such as vitamin C, sucrose, and glucose.
Results
Successful acylation of C3G with lauric acid was achieved, as confirmed by HPLC-MS analysis.
Purification of acylated C3G was performed using PHPLC, yielding a dry powder for further analysis.
FTIR analysis confirmed the structural changes in acylated C3G compared to unacylated C3G.
LC-MS analysis provided detailed molecular information about the acylation products.
Stability analysis demonstrated that acylated C3G showed improved stability compared to unacylated C3G under various conditions, indicating its potential as a food colorant additive
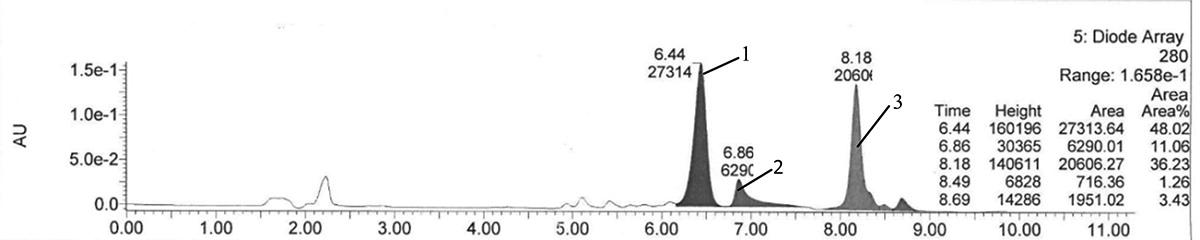 PHPLC chromatogram of products with lauric acids as acyl donor.
PHPLC chromatogram of products with lauric acids as acyl donor.
 Total ion chromatograms of acylated C3G.
Total ion chromatograms of acylated C3G.
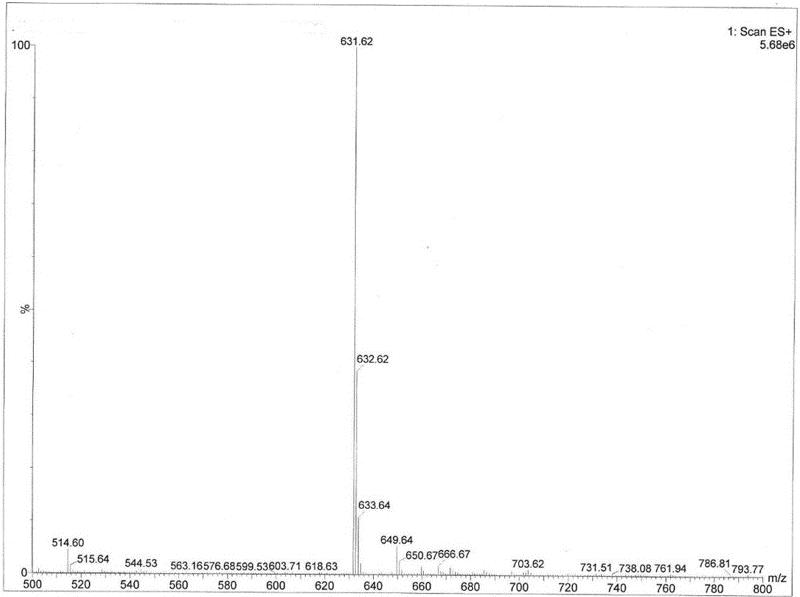 Mass spectrogram of acylated C3G. Note: This was the mass spectrogram of acylated C3G, the m/z was 631.
Mass spectrogram of acylated C3G. Note: This was the mass spectrogram of acylated C3G, the m/z was 631.
Reference
- Zhao, Li-yi, et al. "Direct acylation of cyanidin-3-glucoside with lauric acid in blueberry and its stability analysis." International journal of food properties 19.1 (2016): 1-12.


 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service PHPLC chromatogram of products with lauric acids as acyl donor.
PHPLC chromatogram of products with lauric acids as acyl donor. Total ion chromatograms of acylated C3G.
Total ion chromatograms of acylated C3G. Mass spectrogram of acylated C3G. Note: This was the mass spectrogram of acylated C3G, the m/z was 631.
Mass spectrogram of acylated C3G. Note: This was the mass spectrogram of acylated C3G, the m/z was 631.

