What is Geraniol?
Geraniol is a naturally occurring compound classified as a monoterpenoid alcohol. Geraniol is found in many essential oils, often extracted from natural sources or produced using recombinant microorganisms. It is widely utilized as a fragrance ingredient in cosmetics and household products. Besides its pleasant scent, geraniol has promising potential as a non-toxic and non-irritating transdermal penetration enhancer.
Geraniol analysis is important for accurately identifying and quantifying the presence of geraniol in products or samples. This analysis ensures the quality and consistency of products in industries like cosmetics, food and beverages, and essential oils. It helps in verifying ingredient authenticity, maintaining regulatory compliance, and optimizing formulations for desired sensory properties and effectiveness.
Geraniol Analysis Services by Creative Proteomics
Creative Proteomics provide a range of specialized services for the comprehensive analysis of geraniol. Our expertise includes various analytical techniques designed to deliver precise and detailed information about this important compound. Geraniol analysis falls under the category of our plant metabolomics analysis, allowing us to examine the intricate metabolic pathways and profiles associated with geraniol in plant samples. Below are the specific services and technologies we use:
Geraniol Qualitative Analysis: Using advanced mass spectrometry techniques, we accurately identify the presence of Geraniol in various sample matrices. Confirmation of Geraniol and its related compounds through detailed mass spectrometry data.
Geraniol Quantitative Analysis: Precise measurement of Geraniol concentrations in different sample matrices using validated HPLC and GC methods. Determination of Geraniol content with high precision and accuracy, supporting regulatory compliance and quality control.
Geraniol Physical & Chemical Testing: Analysis of physical properties (e.g., viscosity, density) and chemical properties (e.g., pH, purity) to ensure product quality and consistency.
Geraniol Elemental Analysis: Identifying and quantifying elemental components within geraniol products to ensure they meet safety and regulatory standards.
Batch Analysis and Impurity Profiling: Testing multiple batches of geraniol to assess consistency and identify any impurities present.
Storage Stability: Evaluating how geraniol and its formulations maintain stability over time under various storage conditions.
Techniques and Instrumentation for Geraniol Analysis
Gas Chromatography (GC): GC, using instruments like the Agilent 7890B GC system, analyzes geraniol with high resolution and sensitivity, enabling precise measurement and identification.
High-Performance Liquid Chromatography-Mass Spectrometry (HPLC-MS): HPLC-MS, utilizing devices such as the Agilent 1290 Infinity LC coupled with the Agilent 6460 Triple Quadrupole MS, combines HPLC and mass spectrometry for accurate quantification and detailed identification of geraniol.
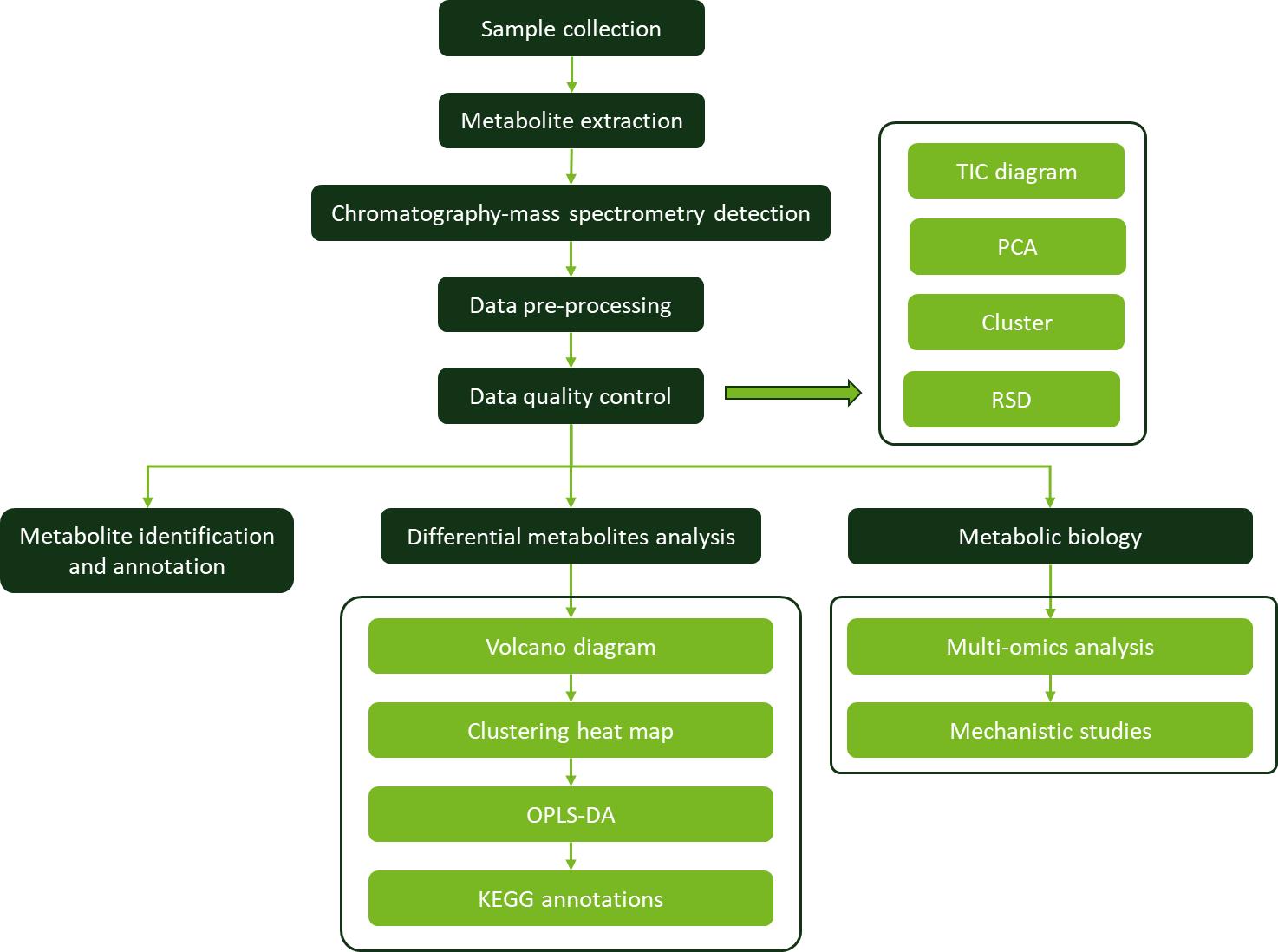
Applications of Geraniol Analysis
Occupational and Environmental Health: Analyzing geraniol in biological samples such as urine is crucial for assessing exposure levels in individuals working in industries like perfume, cosmetics, and food production.
Pharmacokinetics and Metabolism Studies: Identifying specific metabolites like 8-carboxygeraniol as biomarkers through geraniol analysis aids in understanding the pharmacokinetics and metabolic pathways of geraniol in the human body.
Toxicological Studies: By analyzing the concentration of geraniol and its metabolites, researchers can evaluate potential toxic effects and the safety profile of this compound.
Product Formulation and Quality Control: Rigorous geraniol analysis ensures the correct concentration of geraniol in products like perfumes, essential oils, and food items. It also helps in detecting impurities and verifying the purity of geraniol in raw materials and final products, ensuring high-quality standards in product formulation.
Food and Flavor Industry: In the food and flavor industry, geraniol analysis is important for ensuring the desired sensory qualities in food and beverages by analyzing it as a flavor compound. It also helps study the stability of geraniol in food products, impacting shelf life and quality, and ensuring product consistency and safety.
Sample Requirements for Geraniol Analysis
| Sample Types |
Recommended Sample Amount |
| Plant Samples |
Fresh Plant Material |
50-100 g |
| Dried Plant Material |
10-20 g |
| Plant Tissues (e.g., leaves, stems) |
50-100 g |
| Plant Extracts |
1-5 mL |
| Liquid Samples |
Plant Oils |
1-2 mL |
| Plant-Based Formulations |
1-5 g or mL |
| Special Samples |
Complex Formulations |
1-5 g or mL |
| Encapsulated or Solid Samples |
1-5 g |
Q1: What types of samples can you analyze for geraniol content?
A1: We can analyze a wide range of samples for geraniol content, including plant extracts, essential oils, cosmetic products, food and beverage items, and biological samples. Our advanced techniques are versatile and can be applied to various matrices.
Q2: What is the limit of quantification (LOQ) for geraniol in your analysis?
A2: The Limit of Quantification (LOQ) for geraniol depends on the method and instrumentation used. It is the lowest concentration at which geraniol can be reliably quantified with acceptable precision and accuracy. In HPLC, the LOQ is determined through method validation and influenced by detector sensitivity, sample preparation quality, and the calibration curve. For geraniol, the LOQ typically ranges from 0.01 to 0.1% by weight or volume, varying based on the analytical setup.
Q3: How do you handle the storage and transportation of samples for geraniol analysis?
A3: Samples for geraniol analysis are collected using specialist equipment and stored in waterproof and inert vials to ensure their integrity and stability. We provide detailed instructions for sample collection and transportation to maintain sample quality from collection to analysis.
Case. Metabolic Analysis Reveals Altered Sulfur Metabolism in astol1 Mutant Rice under Arsenite Tolerance
Background
Geraniol (trans-3,7-dimethyl-2,6-octadiene-1-ol) is an acyclic isoprenoid monoterpene widely used in fragrances, agrochemicals, and pharmaceuticals. It is prone to autoxidation, forming oxidation products with varying sensitizing potencies, making it a recognized contact allergen. Due to its extensive use, there's a need for effective human biomonitoring (HBM) methods to assess exposure and potential health risks. This study aims to improve and validate a sensitive LC-MS/MS method for analyzing geraniol metabolites, specifically 8-carboxygeraniol and Hildebrandt acid, and apply it to urine samples collected over 14 years to monitor exposure trends.
Samples
The study analyzed 250 24-hour urine samples collected from 2004 to 2018. The samples were derived from 20 to 29-year-old students, with an equal male to female ratio, and were cryo-stored over liquid nitrogen.
Technical Methods Procedure
Sample Preparation:
1 mL of urine spiked with 10 μL of an internal standard mix.
Added 0.5 mL acetate buffer (pH 5.1) and 10 μL enzyme suspension (β-glucuronidase and arylsulfatase).
Incubated at 37 °C for 3 hours. Liquid-liquid extraction with 50 μL phosphoric acid and 2 mL diethyl ether.
LC-MS/MS Analysis:
Organic phase evaporated to dryness and reconstituted in water with 0.033% formic acid.
20 μL injected into a U(H)PLC Nexera X2 system coupled to an MS 6500+ QTRAP. Chromatographic separation on an ACQUITY UPLC HSS T3 column with a gradient elution.
LC-MS/MS performed with positive electrospray ionization in multiple-reaction monitoring mode.
Validation and Performance:
Intra- and inter-day precisions <9% CV, accuracy between 89-113%.
Mean recovery rates 60%, with no matrix suppression for Hildebrandt acid and 20% for 8-carboxygeraniol.
Linear range of 0.5–100 ng/mL for 8-carboxygeraniol, quadratic regression 0.5–800 ng/mL for Hildebrandt acid.
Results
Detection of both metabolites was successful in all 250 urine samples, with average levels of 604.0 μg/24h for Hildebrandt acid and 23.99 μg/24h for 8-carboxygeraniol.
8-carboxygeraniol was identified as the specific biomarker for geraniol exposure, while Hildebrandt acid, though abundant, was not specific.
Females exhibited higher excretion of 8-carboxygeraniol compared to males from 2004 to 2012, with no significant differences in 2015 and 2018.
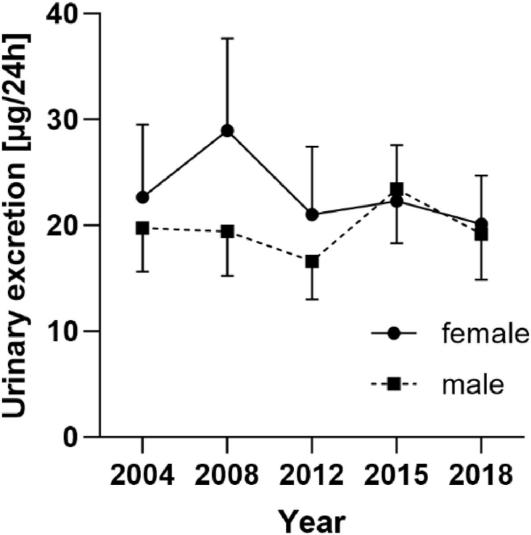 Time course of the urinary excretion (μg/24h) of 8-carboxygeraniol in female and male participants (geometric mean ± 95% CI)
Time course of the urinary excretion (μg/24h) of 8-carboxygeraniol in female and male participants (geometric mean ± 95% CI)
Reference
- Nikola Pluym, et al. (2022) "Time trend of the exposure to geraniol in 24-h urine samples derived from the German Environmental Specimen Bank from 2004 to 2018." International Journal of Hygiene and Environmental Health 239:113880



 Time course of the urinary excretion (μg/24h) of 8-carboxygeraniol in female and male participants (geometric mean ± 95% CI)
Time course of the urinary excretion (μg/24h) of 8-carboxygeraniol in female and male participants (geometric mean ± 95% CI)



