What are Plant Hormones?
Plant hormones are organic compounds produced by plants in minute quantities, influencing growth, development, and responses to biotic and abiotic stressors. These phytohormones act as chemical messengers, facilitating communication between different parts of the plant. The major plant hormones include auxins, cytokinins, gibberellins, abscisic acid (ABA), ethylene, and brassinosteroids, each playing unique roles in plant physiology.
Plant Hormones Analysis Service Provided by Creative Proteomics
Quantitative Analysis of Plant Hormones: We utilize cutting-edge mass spectrometry-based techniques to quantify plant hormone levels accurately. Our services cover a wide range of plant hormones, including auxins, cytokinins, gibberellins, abscisic acid (ABA), ethylene, and brassinosteroids.
Hormone Profiling in Response to Environmental Stress: Creative Proteomics can analyze plant hormone responses to environmental stressors such as drought, salinity, temperature fluctuations, and pathogen attacks. Understanding these stress-induced hormonal changes helps in devising strategies for stress tolerance and resilience in crops.
Analysis of Hormone Interactions and Signaling Pathways: Our expertise includes investigating the intricate crosstalk and interactions between different plant hormones. This analysis sheds light on complex hormonal signaling networks that regulate various physiological processes in plants.
Hormone Analysis in Transgenic Plants: We specialize in analyzing hormone profiles in genetically modified plants. This service enables researchers to assess the impact of genetic alterations on hormone regulation, helping in the development of improved plant varieties.
Customized Plant Hormone Analysis: We understand that every research project is unique. Therefore, we offer customized plant hormone analysis services tailored to the specific needs and objectives of our clients.
Plant Hormones Analysis Techniques and Instrument
Liquid Chromatography-Mass Spectrometry (LC-MS): We utilize advanced LC-MS systems, such as the Thermo Fisher Q Exactive series and Waters Xevo series. LC-MS separates plant hormones based on their chemical properties and ionizes them for mass spectrometric detection, ensuring high-resolution and precise quantification.
Gas Chromatography-Mass Spectrometry (GC-MS): For the analysis of volatile plant hormones like ethylene, we use state-of-the-art GC-MS systems, such as the Agilent 7890B model. GC-MS separates hormone compounds based on their volatility, followed by mass spectrometric identification.
 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service
List of Plant Hormones Analyzed (including but not limited to)
| Types |
Compounds |
| Auxins |
Indole-3-acetic acid (IAA), Indole-3-butyric acid (IBA), 1-Naphthaleneacetic acid (NAA) |
| Cytokinins |
Zeatin, Isopentenyladenine (IPA), N6-(Δ2-Isopentenyl)adenine-9-riboside (iPR) |
| Gibberellins |
Gibberellic acid (GA₁), Gibberellic acid (GA₃), Gibberellin A₄ (GA₄), Gibberellin A₇ (GA₇) |
| Abscisic Acid (ABA) |
Abscisic acid (ABA) |
| Ethylene |
Ethylene |
| Brassinosteroids |
Brassinolide, Castasterone, Brassinosteroid C (CS) |
| Jasmonates |
Jasmonic acid, Jasmonoyl-isoleucine (JA-Ile) |
| Salicylic Acid (SA) |
Salicylic acid |
| Strigolactones |
Strigolactone, Strigol |
| Polyamines |
Putrescine, Spermidine, Spermine |
| Nitric Oxide (NO) |
Nitric oxide |
| Other Compounds |
Indole-3-acetamide (IAM), Indole-3-acetonitrile (IAN) |
Sample Requirements for Plant Hormones Assay
| Sample Types |
Minimum Sample Size |
| Plant Samples |
Roots, stems and leaves, floral parts, fruits/seeds, rhizomes, buds/tender leaves, tissue sections, pollen, bark, trunk/wood, resin/gum, resin acids, seedlings/young plants, rhizosphere soil, root exudates. |
100 mg - 1 g |
| Animal Samples |
Tissues |
100 mg - 1 g |
| Cell Samples |
Cells and Culture |
106 - 108 cells |
Analyzing Plant Hormones Can Help:
1. Optimizing Crop Yield and Quality
Plant hormone analysis enables researchers and agronomists to better understand the hormonal regulation of plant growth and development. By fine-tuning hormone levels, it becomes possible to optimize crop yield and quality. For instance, altering the balance of auxins and cytokinins can promote root and shoot growth, leading to improved crop productivity. Additionally, manipulating gibberellin levels can influence flowering and fruiting, resulting in crops with desirable characteristics, such as larger fruits or longer shelf life.
2. Stress Tolerance and Resilience
Environmental stresses, such as drought, salinity, extreme temperatures, and pathogen attacks, pose significant challenges to agriculture and natural ecosystems. Through plant hormone analysis, researchers can identify hormonal responses that enable plants to withstand and adapt to these stresses. Understanding how plants regulate stress-related hormones like abscisic acid (ABA) and ethylene can pave the way for developing stress-tolerant crop varieties and implementing sustainable agricultural practices in challenging environments.
3. Reducing Chemical Inputs in Agriculture
Analyzing plant hormones can contribute to reducing the reliance on synthetic chemicals in agriculture. By harnessing the hormonal signaling pathways, it is possible to develop eco-friendly plant growth regulators and natural methods for pest and disease control. This approach promotes environmentally sustainable farming practices, minimizing the negative impact of chemical inputs on ecosystems and human health.
4. Accelerating Plant Breeding Programs
Plant hormone analysis provides valuable information for plant breeders to select desirable traits in crop breeding programs. By understanding the hormonal basis of various traits, breeders can develop new varieties that exhibit improved characteristics, such as drought resistance, disease resistance, and higher nutrient content. This accelerates the process of developing improved crops and contributes to global food security.
5. Advancing Plant Biotechnology
Plant hormone analysis is fundamental to plant biotechnology applications, such as tissue culture and genetic engineering. Precise control of hormone levels is essential for successful plant regeneration and transformation. By optimizing hormone formulations, researchers can enhance the efficiency of tissue culture protocols and improve the production of genetically modified plants with desired traits.
6. Conservation and Restoration Efforts
Endangered plant species often face challenges in their propagation and reintroduction into their natural habitats. Plant hormone analysis aids in understanding the hormonal regulation of germination, growth, and development in these species. This knowledge can be used to develop specialized propagation methods, ensuring successful conservation and restoration efforts.
7. Phytoremediation and Environmental Cleanup
Phytoremediation is a green technology that uses plants to remove pollutants and contaminants from the environment. Plant hormone analysis helps in understanding the roles of hormones in this process, allowing researchers to optimize plant selection and engineering for efficient phytoremediation. By harnessing the natural detoxifying abilities of plants, we can mitigate pollution and restore contaminated sites.
8. Biological Research and Fundamental Science
Beyond agricultural applications, plant hormone analysis is of significant interest to fundamental biological research. Studying hormonal interactions and signaling pathways provides insights into basic plant physiology and developmental processes. This knowledge contributes to a deeper understanding of life on Earth and the intricate relationships between plants and their environment.
Case 1. Role of Abscisic Acid in Plant Response to Moderate Dehydration Stress: Transcriptome Analysis and Hormone Profiling in Arabidopsis
Background:
The study focuses on understanding the plant response to moderate dehydration stress and the role of endogenous abscisic acid (ABA) in regulating this stress response. It aims to identify the temporal changes in hormone profiles and gene expression patterns during dehydration stress in Arabidopsis plants.
Samples:
Arabidopsis thaliana ecotype Col-0 (wild-type) and the NCED3 knockout mutant (nced3-2) were used as the plant materials. The nced3-2 mutant has reduced ABA biosynthesis, providing a useful comparison to study ABA's role in the stress response.
Methods:
Plant Growth and Dehydration Stress: Plants were grown in soil tablets and subjected to moderate dehydration stress by withholding water for specific time points (3 to 72 hours). The soil moisture levels were carefully controlled to achieve a specific water content.
Transcriptome Analysis using Oligo DNA Array: Total RNA was extracted from the plants after different durations of dehydration stress. Microarray analysis was conducted using the Agilent Arabidopsis 4 Oligo Microarray, which allowed the identification of genes that responded to dehydration stress. Gene expression data were analyzed using GeneSpring GX software.
Quantitative Real-Time PCR Analysis: qRT-PCR was performed to validate the gene expression data obtained from the microarray. Gene-specific primers were designed, and mRNA levels were normalized using reference genes.
Plant Hormone Profiling: Hormones, including ABA, JA, SA, tZ, and GA4, were extracted and purified from the plant materials using acetonitrile-based extraction. LC-ESI-MS/MS was used to measure the hormone levels in the samples.
Results
The study revealed a bi-phasic response to dehydration stress, consisting of an early and a late response. During the early phase, ABA accumulation induced stomatal closure to reduce water loss. In the late phase, accumulated ABA activated cellular signals to induce protective proteins and metabolites for dehydration tolerance. The ABA biosynthetic mutant nced3-2 showed reduced ABA levels and altered gene expression in response to dehydration stress.
Furthermore, the temporal expression patterns of key genes involved in ABA biosynthesis and signaling, as well as other hormones such as JA, SA, tZ, and GA, were identified. The study also highlighted the involvement of AP2/ERF transcription factors in the early dehydration stress response.
Overall, the findings shed light on the dynamic regulatory mechanisms of plant responses to moderate dehydration stress, with ABA playing a central role in coordinating the stress response pathways.
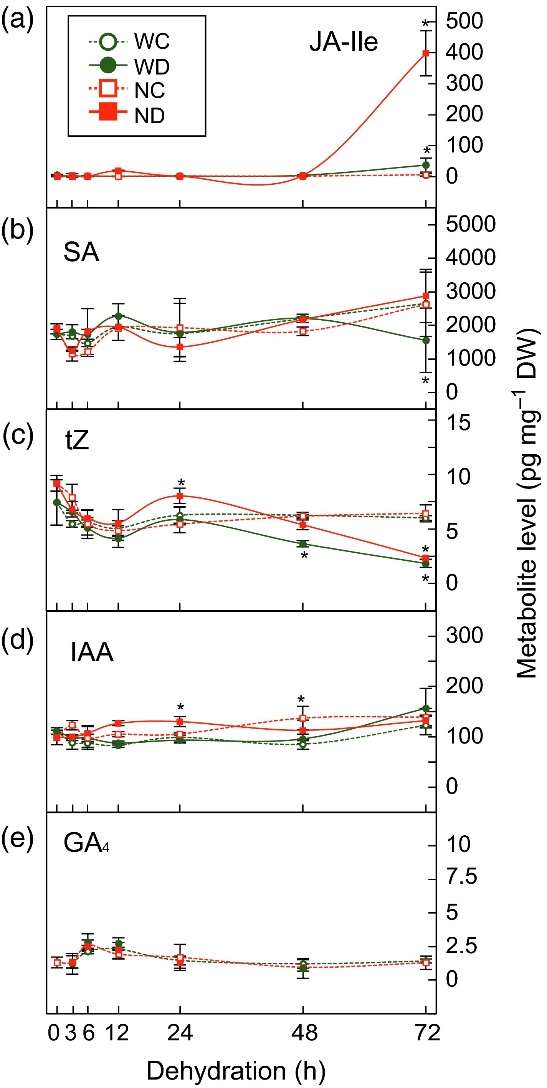 Temporal changes in plant hormone levels in WT and nced3-2 plants in response to moderate dehydration stress.
Temporal changes in plant hormone levels in WT and nced3-2 plants in response to moderate dehydration stress.
Reference
- Urano, Kaoru, et al. "Analysis of plant hormone profiles in response to moderate dehydration stress." The Plant Journal 90.1 (2017): 17-36.


 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service Temporal changes in plant hormone levels in WT and nced3-2 plants in response to moderate dehydration stress.
Temporal changes in plant hormone levels in WT and nced3-2 plants in response to moderate dehydration stress.

