What is Linalool?
Linalool, known chemically as 3,7-dimethyl-1,6-octadien-3-ol or 2,6-dimethyl-2,7-octadien-6-ol, is a naturally occurring acyclic monoterpenoid alcohol with a pleasant floral aroma. It is present in over 200 species of plants, particularly those belonging to the families Lamiaceae (mints and scented herbs), Lauraceae (laurels, cinnamon, rosewood), and Rutaceae (citrus fruits). Linalool has two enantiomers: (R)-(−)-linalool, also known as licareol, and (S)-(+)-linalool, known as coriandrol, both of which are found in various essential oils such as those from coriander (Coriandrum sativum) seeds, lavender (Lavandula officinalis), and sweet basil (Ocimum basilicum).
Linalool analysis is crucial for understanding its concentration and purity in various applications, such as in fragrances, pharmaceuticals, and food products. By accurately analyzing linalool, we can ensure its quality and efficacy, monitor its stability, and adhere to industry standards. This analysis also helps in identifying any potential contaminants or degradation products, thereby optimizing product safety and performance.
Linalool Analysis Services Provided by Creative Proteomics
Qualitative Analysis of Linalool: The analysis is based on the characteristic chromatographic and mass spectral profiles of linalool.
Quantitative Analysis of Linalool: This service employs both GC-MS and LC-MS techniques to accurately measure the concentration of linalool.
Identification of Linalool Isomers: This service utilizes advanced techniques such as GC-MS and LC-MS to separate and identify different isomeric forms of linalool, including (R)-(−)-linalool and (S)-(+)-linalool.
Linalool Purity Assessment: Using GC-MS and LC-MS, this service assesses the presence of impurities or contaminants in linalool samples.
Linalool Release and Absorption Studies: Examining the release and absorption kinetics of linalool in different matrices, such as cosmetic formulations or controlled-release systems. GC-MS and LC-MS are employed to track the release rate and absorption profile of linalool.
Techniques and Instrumentation for Linalool Analysis
GC-MS Methodology
GC Separation: The separation is achieved based on the volatility and interaction of linalool with the column's stationary phase. The temperature gradient helps to separate linalool from other components.
Mass Spectrometry (MS) Detection: The mass spectrum provides a fingerprint of the analyte, allowing for its identification and quantification.
GC-MS Instrumentation
Gas Chromatograph: Equipped with an autosampler, programmable temperature control, and a non-polar capillary column.
Mass Spectrometer: Includes an ionization source (EI or CI), a mass analyzer (quadrupole, ion trap, or time-of-flight), and a detector (electron multiplier or ion detector).
Data Acquisition System: Software for processing and interpreting the chromatographic and mass spectrometric data.
LC-MS Methodology
Liquid Chromatography (LC) Separation: The separation is achieved based on the interaction of linalool with the stationary phase and the mobile phase, which is typically a mixture of water and organic solvents.
Mass Spectrometry (MS) Detection: The ions produced are analyzed based on their mass-to-charge ratio. The resulting mass spectra provide information on the molecular weight and structure of linalool.
LC-MS Instrumentation
Liquid Chromatograph: Includes a high-pressure pump, autosampler, and column oven. The column is typically a reversed-phase C18 column for linalool analysis.
Mass Spectrometer: Features an ionization source (ESI or APCI), a mass analyzer (quadrupole, ion trap, or time-of-flight), and a detector (electron multiplier or ion detector).
Data Acquisition System: Software for chromatographic and mass spectrometric data analysis and interpretation.
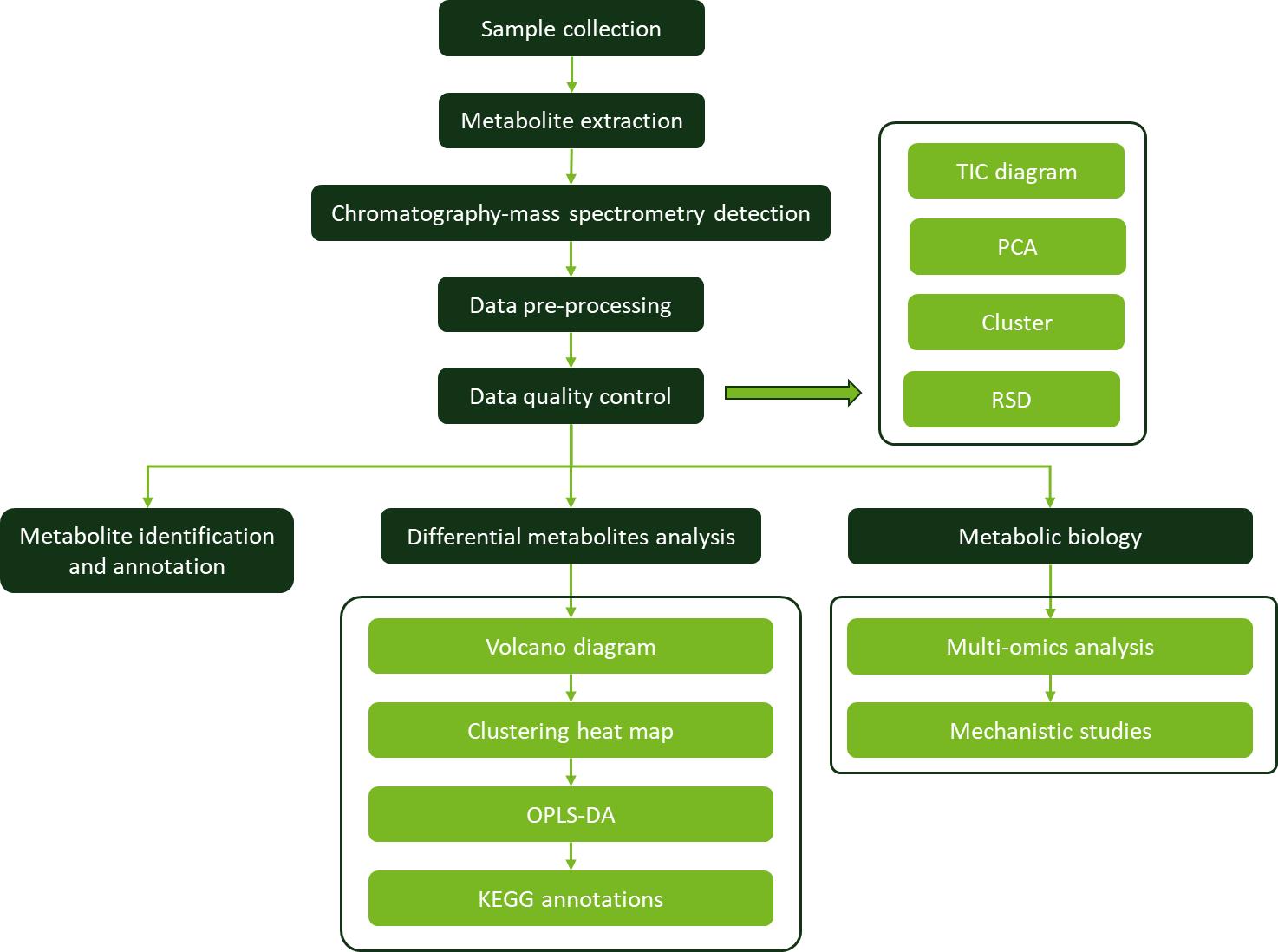
Applications of Linalool Analysis
Pharmaceutical Industry: Ensuring the quality and consistency of pharmaceutical formulations.
Plant-Environment Interaction: Assessing environmental exposure and impact of linalool.
Fragrance and Flavor Industry: Ensuring the quality and consistency of fragrance and flavor formulations.
Personal Care Products: Safety and efficacy testing of cosmetics and personal care products.
Food and Beverage Industry: Quality control and safety testing of food and beverage products.
Sample Requirements for Linalool Analysis
| Sample Types |
Sample Size |
Biological Repeat |
| Plant Samples |
Essential oils, leaves, flowers, seeds |
≥ 1 g (fresh weight) or 50 mg (dried weight) |
3-6 |
| Animal Samples |
Blood, urine, tissue samples (if analyzing linalool metabolites) |
≥ 1 mL (liquid) or 50 mg (tissue) |
| Cell Samples |
Cell culture supernatants, lysates |
≥ 1 million cells or 1 mL of cell lysate |
Q1: Can you provide references or case studies of previous linalool analysis projects?
A1: Yes, we can provide references or case studies upon request. These examples demonstrate our expertise and the quality of our linalool analysis services. Please contact us to receive relevant case studies or references that align with your specific interests and needs.
Q2: What kind of report will I receive, and what information will it include?
A2: You will receive a comprehensive report detailing the findings of the linalool analysis. The report includes information such as the concentration of linalool, any detected impurities, and a summary of the analytical methods used. Detailed results, including data tables and chromatograms, will also be provided. The format and level of detail can be customized based on your needs.
Case. Mutational Analysis of Linalool Dehydratase Isomerase Suggests That Alcohol and Alkene Transformations Are Catalyzed Using Noncovalent Mechanisms
Background
The study investigates the structure and activity of the enzyme LinD, focusing on its interactions with β-myrcene and linalool. LinD, a member of the terpene synthase family, is known for its role in catalyzing the (de)hydration and isomerization of terpenes. The research aims to elucidate the mechanism of LinD by studying its crystal structures in complex with various ligands, preparing and analyzing a library of mutants, and exploring kinetic parameters.
Samples
Wild-Type LinD (wt-LinD): The enzyme was expressed and purified for crystallographic and activity assays.
Ligands: β-Myrcene and linalool, used for complex formation with LinD.
Mutants: A series of site-directed mutants including C171A, C180A, C171S, D39A, Y45F, M125A, E172A, H129A, and Y66F, designed to investigate specific residues' roles in LinD activity.
Technical Methods Procedure
Crystallography: LinD crystals were obtained by co-crystallization with racemic linalool.
Mutation and Purification: Site-directed mutants were created and purified using nickel-affinity chromatography and gel filtration.
Kinetic Analysis: Kinetic constants (Km and kcat) for wt-LinD and selected mutants were determined using GC assays.
Mass Spectrometry: Mass spectrometry was used to detect potential thioterpene intermediates and confirm the lack of covalent modification in cysteine residues.
1H-NMR: NMR spectroscopy was employed to observe the evolution of product peaks and validate proton transfer during reactions.
Results
The structure of wt-LinD complexed with β-myrcene revealed improved electron density and defined conformation of β-myrcene.
Structural studies of mutants revealed conformational changes but no significant ligand density indicating intermediate binding.
The roles of specific residues (D39, Y45, M125, H129, and Y66) in catalysis were elucidated, supporting the hypothesis of protonation and hydration mechanisms.
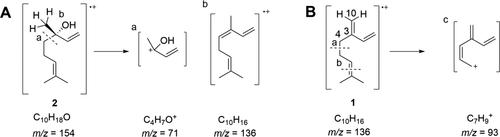 Fragmentation of (A) β-myrcene and (B) (S)-linalool in MS analysis.
Fragmentation of (A) β-myrcene and (B) (S)-linalool in MS analysis.
Reference
- Cuetos, A., et al. (2020). Mutational analysis of linalool dehydratase isomerase suggests that alcohol and alkene transformations are catalyzed using noncovalent mechanisms. ACS Catalysis, 10(19), 11136-11146.



 Fragmentation of (A) β-myrcene and (B) (S)-linalool in MS analysis.
Fragmentation of (A) β-myrcene and (B) (S)-linalool in MS analysis.



