What is Fisetin?
Fisetin, scientifically known as 3,3',4',7-Tetrahydroxyflavone, is a plant polyphenol from the flavonoid group. It is present in various fruits and vegetables such as strawberries, apples, persimmons, onions, and cucumbers. Fisetin has recently gained considerable attention due to its potent antioxidative, anti-inflammatory, antihyperglycemic properties, and potential anti-cancer effects.
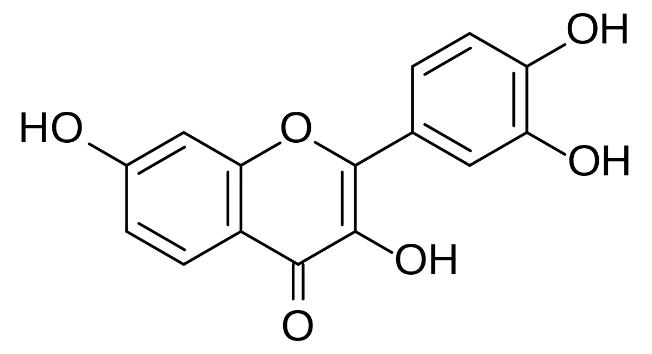 Fisetin
Fisetin
Being a potent bioactive compound, fisetin proves useful in various applications such as nutritional supplements and cosmetics. The elucidation of fisetin's potential therapeutic applications necessitates its accurate identification and quantification in various matrices. This makes fisetin analysis an indispensable tool in providing insightful data on this bioactive compound.
At Creative Proteomics, we boast of our expertise in fisetin analysis.
Specific Fisetin Analysis Offered by Creative Proteomics
Identification of Fisetin Derivatives: Our advanced analytical techniques are adept at identifying various fisetin derivatives. Through rigorous analysis, we unveil the distinct properties and characteristics of these derivatives, providing valuable insights for further research and development.
Quantitative Fisetin Analysis: Creative Proteomics excels in quantitative analysis, allowing for precise measurement of fisetin concentrations in different samples. Whether you are investigating the bioavailability of fisetin or assessing its levels in specific tissues, our quantitative analysis provides accurate and reproducible results.
Structural Characterization: Unraveling the molecular structure of fisetin and its derivatives is essential for understanding their functional attributes. Our fisetin analysis services include structural characterization, employing cutting-edge technologies to elucidate the intricate details of the compound's composition.
Comparative Analysis: For projects requiring a comparative approach, Creative Proteomics conducts thorough comparative analyses of fisetin and related compounds. This facilitates a deeper understanding of the compound's uniqueness and its potential advantages in various applications.
Formulation Studies: In the pharmaceutical and nutraceutical industries, the formulation of fisetin-containing products is a critical aspect. Creative Proteomics offers specialized services to study the compatibility of fisetin with different formulations, ensuring optimal stability and efficacy.
Bioavailability Assessment: Understanding how fisetin is absorbed and utilized by the body is crucial for its potential therapeutic applications. Our services include bioavailability assessments, providing valuable data for the development of fisetin-based pharmaceuticals or dietary supplements.
Toxicological Studies: For a comprehensive evaluation of fisetin's safety profile, Creative Proteomics conducts toxicological studies. This involves assessing the potential adverse effects and establishing the compound's safety parameters, contributing to its regulatory compliance.
Fisetin Analysis Techniques
At Creative Proteomics, we employ the use of mass spectrometry-based techniques for fisetin analysis. Mass spectrometry is a versatile tool allowing us to detect, identify, and quantify intricate compounds present in various matrices.
Liquid Chromatography-Mass Spectrometry (LC-MS)
Our state-of-the-art Liquid Chromatography-Mass Spectrometry (LC-MS) systems such as the Agilent 1290 Infinity II LC System and Q Exactive™ hybrid quadrupole-Orbitrap mass spectrometer provide an excellent solution for the specific and sensitive analysis of fisetin. This includes both qualitative and quantitative analysis which aids in the structural elucidation and detection of fisetin in diverse samples.
Gas Chromatography-Mass Spectrometry (GC-MS)
We further use Gas Chromatography-Mass Spectrometry (GC-MS) techniques utilizing the Agilent 7890A GC System combined with a 5975 Inert XL Mass Selective Detector for the rapidly evolving needs of fisetin analysis. This technique is especially suitable for the analysis of volatile and semi-volatile compounds.
 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service
List of Fisetin and Its Derivatives Analyzed (including but not limited to)
| Fisetin |
Fisetin-3-glucoside |
Fisetin-3-galactoside |
Fisetin-4'-glucoside |
| Fisetin-4'-galactoside |
Fisetin-7-glucoside |
Fisetin-7-galactoside |
Fisetin-3,4'-diglucoside |
| Fisetin-3,7-diglucoside |
Fisetin-4'-glucuronide |
Fisetin-7-glucuronide |
|
Sample Requirements for Fisetin Assay
| Sample Type |
Recommended Quantity |
| Plant Extract |
100 mg |
| Serum |
500 µL |
| Tissue Homogenate |
50 mg |
| Dietary Supplement |
200 mg |
| Food Product |
10 g |
Deliverables from Fisetin Analysis
- A detailed report of experimental procedures
- Raw and processed data sheets
- Analysis results complete with graphs
- Professional suggestions based on the results
Case. Pharmacokinetic Study of Fisetin and Its Metabolite Geraldol in Mice
Background
Fisetin, a flavonoid found in various fruits and vegetables, possesses diverse pharmacological activities including antioxidant, anti-inflammatory, and anticancer effects. Geraldol, a metabolite of fisetin, has also been identified as active in vivo. However, pharmacokinetic data on fisetin and geraldol are limited, necessitating further investigation.
Sample
Male ICR mice were used for the pharmacokinetic study. Fisetin was administered to the mice intravenously (2 mg/kg) or orally (100 and 200 mg/kg), and blood samples were collected at specified time points.
Technical Platform and Procedure
Chemicals and Reagents: Fisetin, geraldol, and terfenadine (internal standard) were obtained from specified sources. Plasma sample preparation involved mixing with acetonitrile containing formic acid and internal standard.
LC-MS/MS: Liquid chromatography-tandem mass spectrometry was employed for analysis. A gradient program with solvent A (0.1% formic acid in ACN) and solvent B (0.1% formic acid in water) was utilized. The method involved multiple reaction monitoring (MRM) mode for quantification.
Method Validation: Calibration curves were constructed for fisetin and geraldol. Accuracy, precision, and stability of the method were evaluated. The method demonstrated acceptable performance for quantitative analysis.
Pharmacokinetic Study: A non-compartmental model was used to determine pharmacokinetic parameters including area under the plasma concentration-time curve (AUC), maximum plasma concentration (Cmax), time to reach Cmax (Tmax), and half-life (T1/2).
Results
Fisetin and geraldol concentrations in mouse plasma were successfully quantified using the developed LC-MS/MS method.
Pharmacokinetic parameters were determined for fisetin and geraldol after oral and intravenous administration.
Fisetin was rapidly metabolized to geraldol in vivo, with similar Tmax values for both compounds.
Absolute bioavailability of fisetin ranged from 7.8% to 31.7% after oral administration.
Fisetin metabolism to geraldol was not saturated even at high doses, suggesting high metabolic capacity.
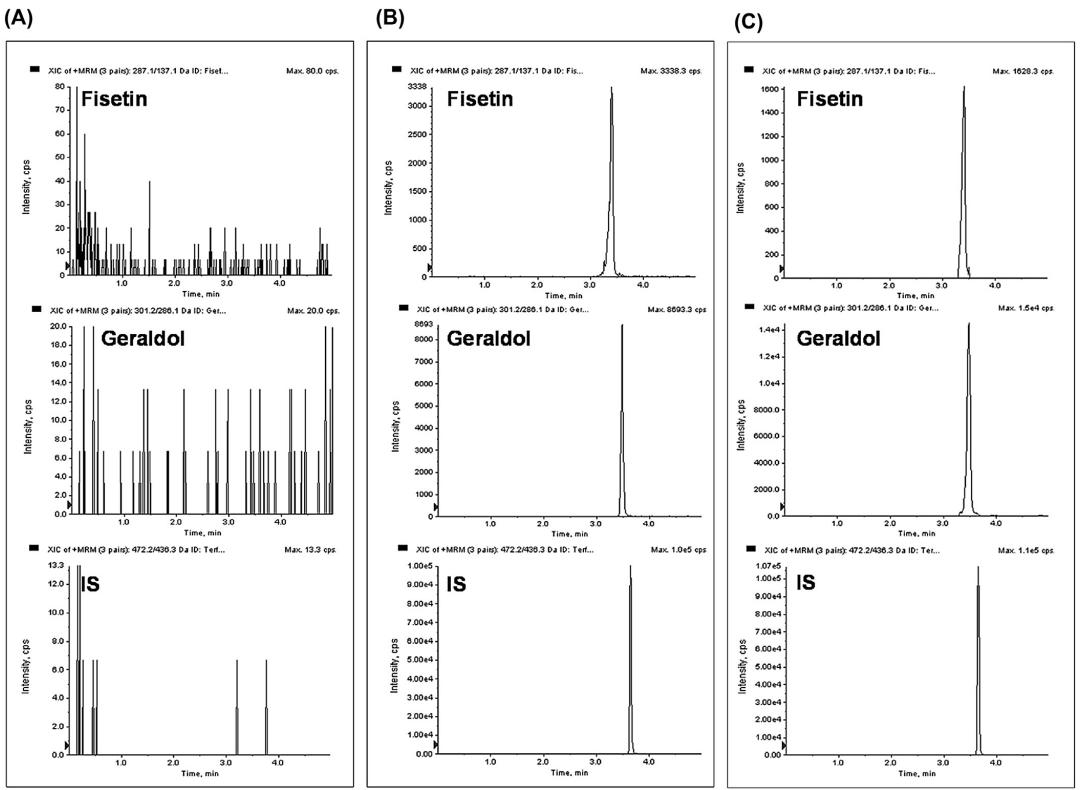 Representative MRM chromatograms of fisetin, geraldol, and terfenadine (internal standard) in blank mouse plasma (A), blank mouse plasma spiked with 2 g/mL of fisetin or geraldol (B) and mouse plasma sample obtained 5 min following intravenous administration of 2 mg/kg fisetin.
Representative MRM chromatograms of fisetin, geraldol, and terfenadine (internal standard) in blank mouse plasma (A), blank mouse plasma spiked with 2 g/mL of fisetin or geraldol (B) and mouse plasma sample obtained 5 min following intravenous administration of 2 mg/kg fisetin.
Reference
- Jo, Jun Hyeon, et al. "Identification of absolute conversion to geraldol from fisetin and pharmacokinetics in mouse." Journal of Chromatography B 1038 (2016): 95-100.


 Fisetin
Fisetin Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service Representative MRM chromatograms of fisetin, geraldol, and terfenadine (internal standard) in blank mouse plasma (A), blank mouse plasma spiked with 2 g/mL of fisetin or geraldol (B) and mouse plasma sample obtained 5 min following intravenous administration of 2 mg/kg fisetin.
Representative MRM chromatograms of fisetin, geraldol, and terfenadine (internal standard) in blank mouse plasma (A), blank mouse plasma spiked with 2 g/mL of fisetin or geraldol (B) and mouse plasma sample obtained 5 min following intravenous administration of 2 mg/kg fisetin.

