Phenolic acids, derivatives of benzoic and cinnamic acids, are characterized by their aromatic ring structures and hydroxyl groups. These compounds are synthesized through the shikimate and phenylpropanoid pathways within plants. Their diverse functions include acting as antioxidants, UV protectants, and defense compounds against pathogens and herbivores.
The metabolic journey of phenolic acids within plants is intricate and involves numerous enzymatic reactions. Creative Proteomics specializes in deciphering these metabolic pathways through advanced metabolomics analyses. Our expertise enables us to identify and quantify an extensive array of phenolic acid metabolites, shedding light on their transformation and biological significance.
Understanding the distribution and abundance of phenolic acids and their metabolites is critical. These compounds contribute to the organoleptic and nutritional quality of plant-derived foods and beverages, influencing their flavor, color, and potential health benefits. In addition, the antioxidant properties of phenolic acids make them attractive targets for therapeutic and nutritional drug development. Through our precision analytical services, Creative Proteomics facilitates comprehensive phenolic acid analysis, enabling researchers and industry to capitalize on the potential of these compounds.
Phenolic Acid Detection Projects at Creative Proteomics
Creative Proteomics offers an extensive repertoire of phenolic acid analysis services that cater to diverse research needs. Our specialized offerings include:
Quantitative Profiling: Employing state-of-the-art liquid chromatography-mass spectrometry (LC-MS) systems, such as the Agilent 1290 Infinity II LC and the Thermo Scientific Q Exactive HF-X, we enable accurate and sensitive quantification of phenolic acids and their metabolites in complex matrices.
Structural Elucidation: Creative Proteomics utilizes high-resolution mass spectrometers, including the Waters SYNAPT G2-Si, to elucidate the chemical structures of phenolic acid compounds. Through tandem mass spectrometry (MS/MS) analyses, we unravel the fragmentation patterns, aiding in precise identification.
Metabolic Pathway Analysis: Leveraging advanced data analysis pipelines, we offer comprehensive insights into the metabolic pathways and networks involving phenolic acids. This information is invaluable for understanding their roles in plant physiology and potential applications.
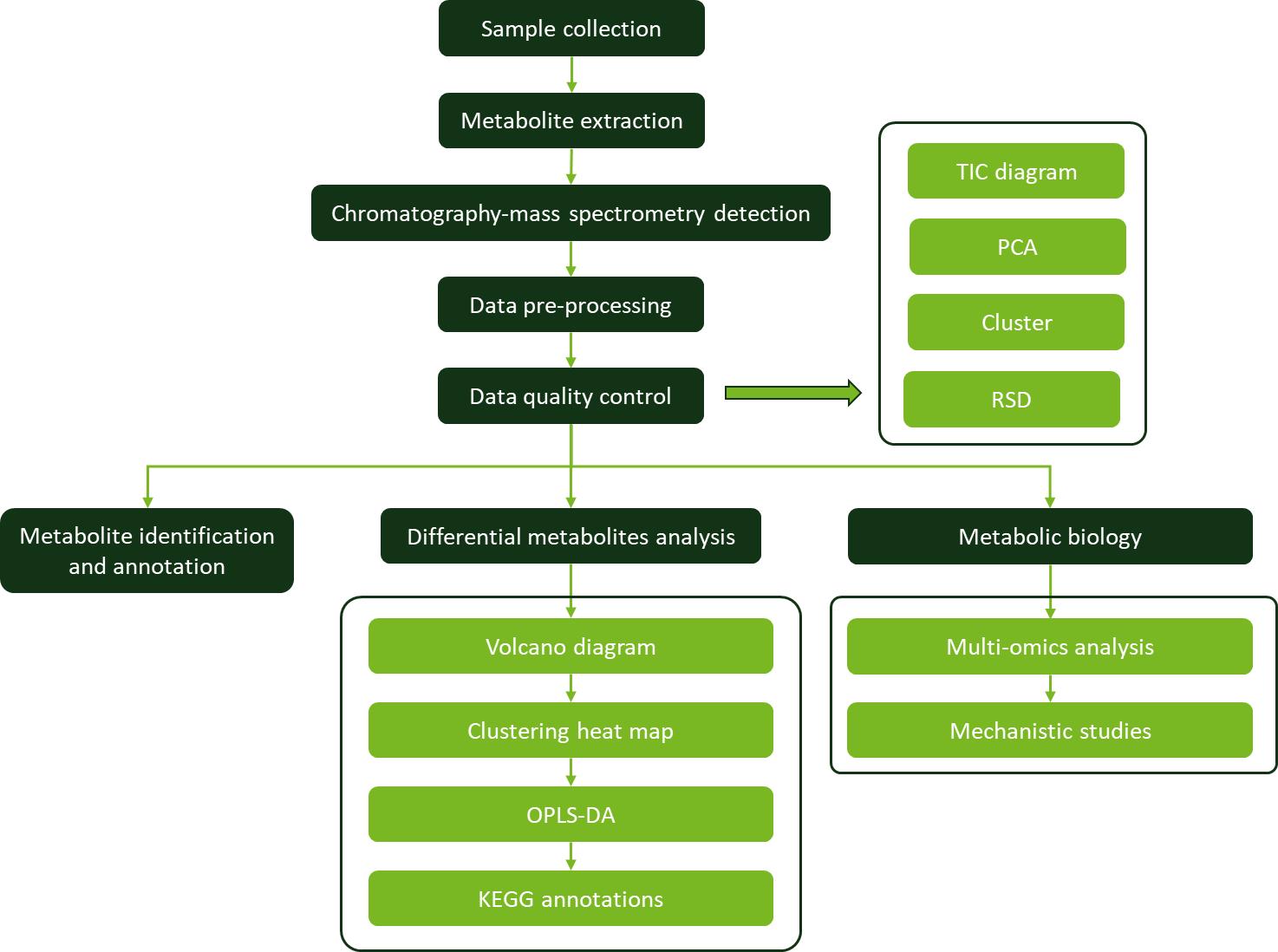 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service
List of Phenolic Acid Compounds Analyzed (including but not limited to)
| 4-Hydroxybenzoic acid |
4-Hydroxycinnamic acid |
Amygdalin |
Apigenin |
Caffeic acid |
| Catechin |
Chlorogenic acid |
Cinnamic acid |
Curcumin |
Ellagic acid |
| Epicatechin |
Ferulic acid |
Feruloylquinic acid |
Gallic acid |
Galloylquinic acid |
| Gentisic acid |
Homoprotocatechuic acid |
Homovanillic acid |
Isorhamnetin |
Kaempferol |
| Lignoceric acid |
Luteolin |
Methyl gallate |
Myricetin |
Naringenin |
| Oroxylin A |
Piceatannol |
p-Coumaric acid |
Protocatechualdehyde |
Protocatechuic acid |
| Protocatechuic aldehyde |
Quercetin |
Resveratrol |
Rosmarinic acid |
Rosamarinic acid |
| Rutin |
Salicylic acid |
Sinapic acid |
Syringaresinol |
Syringic acid |
| Taxifolin |
Tyrosol |
Vanillic acid |
Vanillic alcohol |
Vanillylmandelic acid |
Sample Requirements for Phenolic Acid Assay
| Sample Types |
Minimum Sample Size |
| Plant Samples |
Roots, stems and leaves, floral parts, fruits/seeds, rhizomes, buds/tender leaves, tissue sections, pollen, bark, trunk/wood, resin/gum, resin acids, seedlings/young plants, rhizosphere soil, root exudates. |
50 mg - 1 g |
| Animal Samples |
Urine |
1-2 mL |
| Serum/Plasma |
100-500 µL |
Applications of Phenolic Acid Metabolomics Analysis
Nutraceutical Development: Insights gained from phenolic acid metabolomics guide the formulation of functional foods and supplements, harnessing their potential health benefits.
Plant Breeding: Understanding phenolic acid profiles in different plant varieties aids in breeding for enhanced nutritional content and stress resistance.
Pharmacological Research: Phenolic acids and their metabolites serve as promising candidates for drug development, targeting oxidative stress-related disorders and inflammation.
Environmental Studies: Phenolic acid analysis in environmental samples contributes to assessing plant responses to changing ecosystems and pollution levels.
Case 1. Comprehensive Quantification of Polyphenols in Diverse Plant Materials Using UHPLC-MS/MS Coupled with SLE and SPE Techniques
Background:
The study aimed to develop an effective method for the simultaneous quantification of 30 polyphenols in diverse plant materials using a novel approach combining UHPLC-MS/MS, solid-liquid extraction (SLE), and solid-phase extraction (SPE). Polyphenols, encompassing phenolic acids and flavonoids, are bioactive compounds found in plants, known for their antioxidant and health-promoting properties.
Samples:
The research focused on six plant species: lucerne, licorice, goldenrod, buckwheat, phacelia, and lavender. Various parts of these plants, such as flowers, leaves, stalks, and roots, were analyzed to evaluate the distribution and content of polyphenols in different plant matrices.
Methods:
Sample Preparation:
Six different plant species were selected: lucerne, licorice, goldenrod, buckwheat, phacelia, and lavender.
Various parts of these plants, including flowers, leaves, stalks, and roots, were collected and prepared for analysis.
Extraction Procedure (SLE):
Solid-Liquid Extraction (SLE) was employed to extract polyphenols from the plant samples.
Methanol (MeOH) was chosen as the extraction solvent due to its effectiveness in isolating both phenolic acids and flavonoids.
The plant samples were mixed with MeOH and subjected to shaking for a specific duration.
The resulting extract was then subjected to centrifugation to separate the solid and liquid phases.
Solid-Phase Extraction (SPE):
The MeOH extracts were further purified using Solid-Phase Extraction (SPE) with a C18 reversed-phase cartridge.
The cartridge was conditioned with a specific solvent (e.g., MeOH) before loading the extract.
The extract was passed through the cartridge, retaining polyphenols while removing impurities.
Elution of retained compounds was performed using an appropriate solvent (e.g., MeOH).
UHPLC-MS/MS Analysis:
Ultra-High-Performance Liquid Chromatography tandem Mass Spectrometry (UHPLC-MS/MS) was used for compound separation and quantification.
The purified extracts were injected into the UHPLC system equipped with a suitable column and mobile phase.
Mass spectrometry was employed for compound detection and quantification through Multiple Reaction Monitoring (MRM) mode.
Calibration curves were generated using standard solutions of polyphenolic compounds to determine concentrations.
Validation and Parameters:
Linearity of calibration curves was established for each polyphenol over a specific concentration range.
Limits of quantification (LOQs) were determined to assess the method's sensitivity.
Precision was evaluated by calculating Relative Standard Deviations (RSDs) for intra-day and inter-day accuracy.
Recovery experiments were conducted by spiking known concentrations of standards into plant samples to assess extraction efficiency.
Stability and variability of analytes were assessed by analyzing samples under different conditions.
Data Analysis and Quantification:
Software tools were used to process raw UHPLC-MS/MS data, including peak identification, integration, and quantification.
The obtained quantitative data for polyphenols in different plant parts and species were analyzed statistically.
Application to Plant Analysis:
The validated method was applied to analyze the polyphenolic content in different parts of the selected plant species.
Quantitative data were generated for each identified polyphenol in various plant samples.
Comparison and Interpretation:
The obtained data were compared and interpreted to determine differences in polyphenol profiles among different plant parts and species.
Major phenolic acids and flavonoids in each plant species were identified and quantified.
Results
The results demonstrated that the choice of extraction solvent significantly impacted the polyphenol content in the extracts. Methanol-based extraction, followed by C18 SPE purification, exhibited favorable recoveries and precision for most compounds, with RSDs below 10%. LOQs ranged from 0.4 to 20 ng/mL, highlighting the sensitivity of the method. Application of the developed technique to the plant samples revealed distinct profiles of polyphenols in different plant parts. Major phenolic acids included 3,4-HPPA, 4-HBA, and 3,4-DHBA, while significant flavonoids comprised QUE, RUT, GLB, and NAR. The method's success in analyzing complex plant matrices underscores its potential for quality control and selection of plant materials for various applications.
In summary, this study innovatively combined UHPLC-MS/MS, SLE, and SPE techniques to establish a robust method for the simultaneous quantification of polyphenols in diverse plant materials. The method's efficacy, precision, and sensitivity were demonstrated, enabling comprehensive analyses of polyphenol distribution in different plant parts across multiple species.
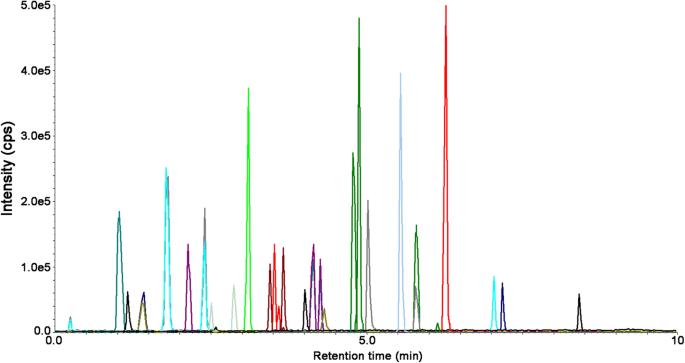 UHPLC-MS/MS chromatogram of a standard solution containing the analyzed polyphenols and IS
UHPLC-MS/MS chromatogram of a standard solution containing the analyzed polyphenols and IS
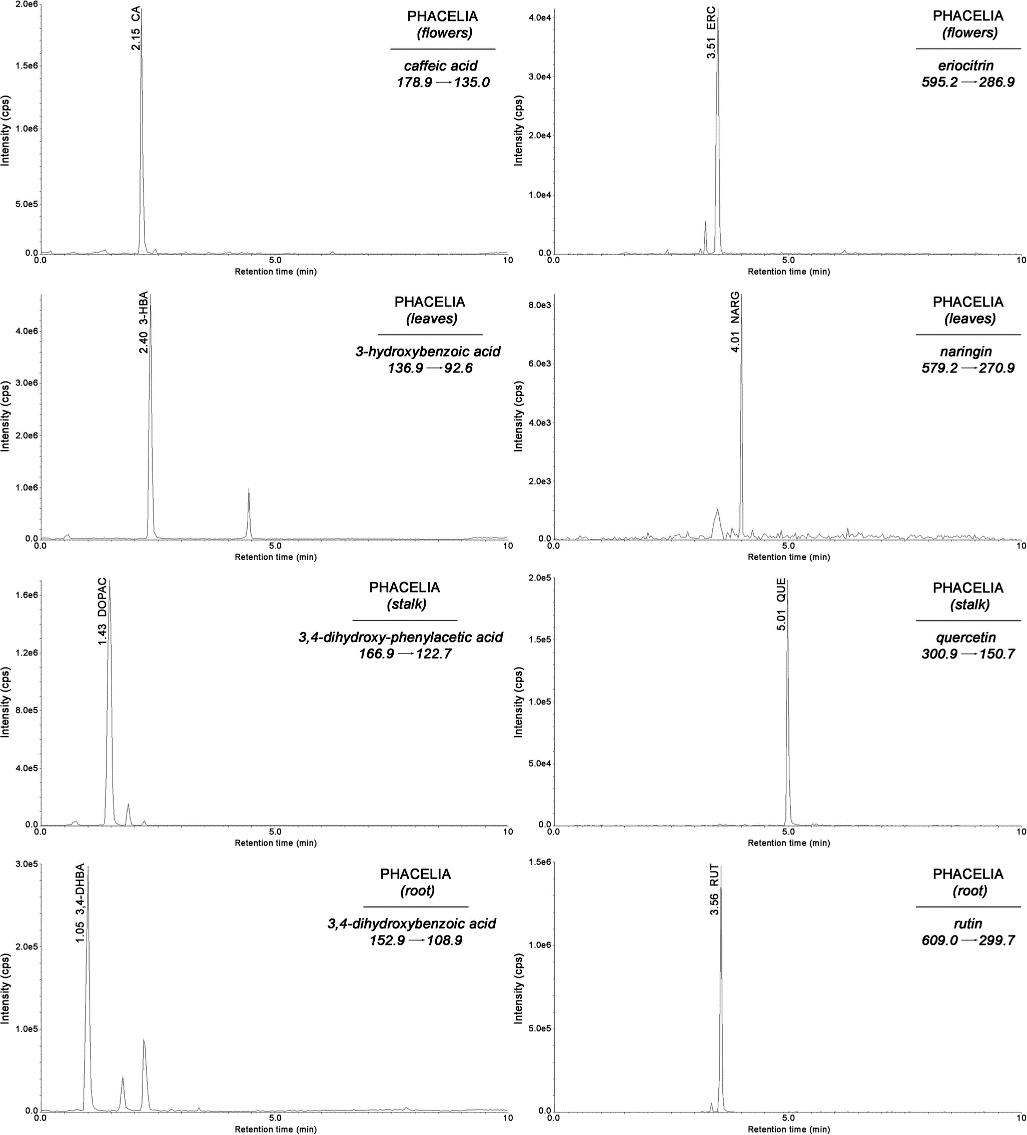 Representative MRM chromatograms of selected phenolic acids and flavonoids analyzed in extracts of phacelia flowers, leaves, stalks, and roots
Representative MRM chromatograms of selected phenolic acids and flavonoids analyzed in extracts of phacelia flowers, leaves, stalks, and roots
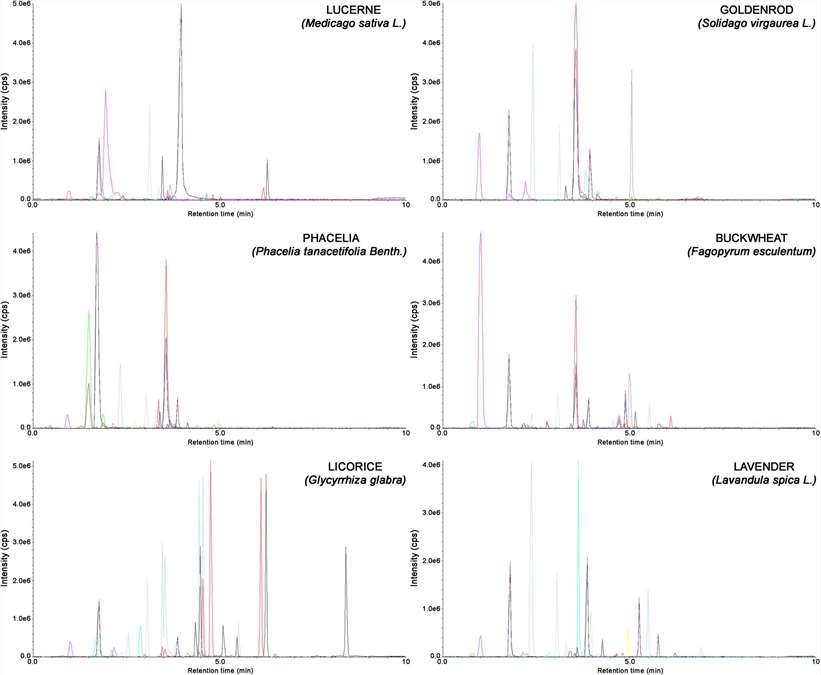 Representative chromatograms obtained for extracts of lucerne, goldenrod, phacelia, buckwheat, licorice, and lavender using the proposed SLE-SPE-UHPLC-MS/MS method
Representative chromatograms obtained for extracts of lucerne, goldenrod, phacelia, buckwheat, licorice, and lavender using the proposed SLE-SPE-UHPLC-MS/MS method
Reference
- Bajkacz, Sylwia, et al. "Determination of flavonoids and phenolic acids in plant materials using SLE-SPE-UHPLC-MS/MS method." Food Analytical Methods 11 (2018): 3563-3575.


 Workflow for Plant Metabolomics Service
Workflow for Plant Metabolomics Service UHPLC-MS/MS chromatogram of a standard solution containing the analyzed polyphenols and IS
UHPLC-MS/MS chromatogram of a standard solution containing the analyzed polyphenols and IS Representative MRM chromatograms of selected phenolic acids and flavonoids analyzed in extracts of phacelia flowers, leaves, stalks, and roots
Representative MRM chromatograms of selected phenolic acids and flavonoids analyzed in extracts of phacelia flowers, leaves, stalks, and roots Representative chromatograms obtained for extracts of lucerne, goldenrod, phacelia, buckwheat, licorice, and lavender using the proposed SLE-SPE-UHPLC-MS/MS method
Representative chromatograms obtained for extracts of lucerne, goldenrod, phacelia, buckwheat, licorice, and lavender using the proposed SLE-SPE-UHPLC-MS/MS method

