Small ubiquitin-related modifier (SUMO) is a conserved, reversible, and dynamic post-translational modification (PTM)
of protein. Over the past 20 years, there have been more than 3,000 SUMOylated proteins identified in cells. This
modification has been found to regulate the activity of proteins and thus affect many intracellular processes
involved in the regulation of cellular physiological and pathological processes. Creative
Proteomics is a leading service provider of PTM analysis. We have launched a powerful and
high-throughput method combining stable isotope labeling by amino acids in cell culture (SILAC)-based quantitative
proteomics and peptide enrichment strategies to analyze SUMOylated proteins and identify SUMOylation sites. Our
services have a wide range of applications, including identifying unknown SUMO targets and characterizing changes in
SUMOylome in response to drugs, toxins, environmental stresses, or bacterial and viral infections, helping
researchers explore the role of SUMOylome in cell physiology and disease.
Overview of protein SUMOylation
SUMOylation refers to the process by which a member of the SUMO family proteins binds to a lysine (Lys) residue in a
target protein. This modified protein can be deSUMOylated by sentrin/SUMO-specific proteases (SENPs), which is
controlled by an enzymatic pathway, similar to the ubiquitination pathway. Unlike ubiquitination, SUMOylation does
not target proteins for protein hydrolysis, but rather regulates protein function in a variety of cellular
processes, such as protein-DNA binding, protein stability, DNA repair, stress response, and cell cycle progression.
In addition, accumulating evidence suggests that abnormal SUMO regulation is highly associated with a variety of
diseases, such as neurodegenerative diseases and cancer. In recent years, SUMOylation has become one of the hotspots
of research as a competitor to ubiquitination.
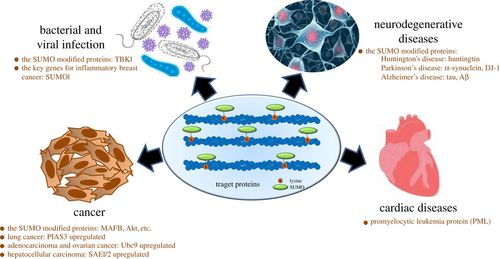 Fig. 1
Relationship of SUMO-modified proteins with different diseases. (Yang, Yanfang, et al., 2017)
Fig. 1
Relationship of SUMO-modified proteins with different diseases. (Yang, Yanfang, et al., 2017)
SUMO proteomics analysis
In order to help researchers and professionals better understand the role of protein SUMOylation modification,
identify proteins modified by SUMO, and understand the exact sites of SUMO conjugation, we offer protein SUMOylation
analysis services, ranging from protein processing, SUMOylated peptide enrichment, LC-MS/MS analysis, to various
bioinformatics analysis. Since the low abundance of most SUMOylated proteins, the identification of SUMOylation has
faced several challenges. Based on experienced experts and optimized workflows, we are able to provide faster and
more sensitive SUMOylation analysis. Our services can help our customers achieve the following lists, including:
- More efficient enrichment of SUMOylated proteins with tandem affinity purification in complex biological
samples.
- Globally analyze SUMOylated proteins and detect SUMOylation sites.
- Allow SUMOylome comparison between two different cell populations.
- Allow general SUMO consensus motif and extended motif characterization.
- Protein SUMOylation site prediction by bioinformatics software analysis.
- SUMO modification usually occurs under specific conditions and therefore needs to be detected on a case-by-case
basis.
Sample requirements
- Acceptable samples: Protein extracts, cell samples, tissue samples, and microorganism samples.
- Sample shipping: Sufficient amount of dry ice for shipping, or consult our technical staff before sending
samples.
- Before the formal experiment, we will always test the samples you provide.
Applications of our service
- Deciphering protein SUMOylation-mediated biological functions.
- Exploration of SUMOylation-mediated molecular mechanisms of disease, such as innate immunity, neurodegenerative
disease, and cancer.
- Signal crosstalk study of SUMOylation and ubiquitination.
- Exploration of SUMOylation of plant proteins.
Our customer service representatives are available 24 hours a day, 7 days a week. Please feel free to contact us for more details.
References
- Yang, Yanfang, et al. "Protein SUMOylation modification and its associations with disease."
Open biology 7.10 (2017): 170167.
- Benlloch, Reyes, and L. Maria Lois. "Sumoylation in plants: mechanistic insights and its role in drought
stress." Journal of experimental botany 69.19 (2018): 4539-4554.
Our products and services are for research use only.

 Fig. 1
Relationship of SUMO-modified proteins with different diseases. (Yang, Yanfang, et al., 2017)
Fig. 1
Relationship of SUMO-modified proteins with different diseases. (Yang, Yanfang, et al., 2017)