The low abundance of many of the modified peptides limits the detection of some post-translational modifications (PTMs) classes by liquid chromatography-tandem mass spectrometry (LC-MS/MS), so strategies to enrich modified peptides are needed to enhance their detection. Creative Proteomics is a leading contract research organization (CRO) with extensive experience in protein PTM analysis. In addition to effective protein extraction methods and advanced mass spectrometers, we also provide a wide range of modified peptide separation and enrichment strategies to facilitate the global analysis of PTMs and the identification of a targeted PTM within the proteome.
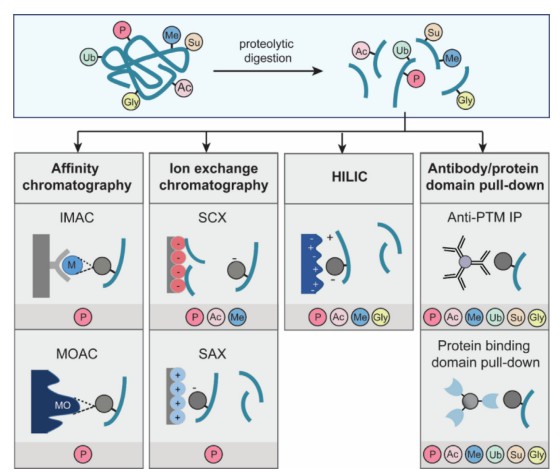 Fig. 1 Summary of the most common affinity enrichment strategies for PTMs analysis. (Brandi, Jessica, et al, 2022)
Fig. 1 Summary of the most common affinity enrichment strategies for PTMs analysis. (Brandi, Jessica, et al, 2022)
Peptide separation and enrichment considerations
PTMs are characterized by substoichiometric, transient and labile. Besides, common PTMs such as phosphorylation, acetylation, ubiquitination, glycosylation, and methylation, each represent distinct types of chemical modifications. To overcome these issues of PTMs, enrichment strategies of the modified peptides of interest have been developed prior to MS analysis, such as ion exchange chromatography (IEC), immunoaffinity precipitation, and immobilized metal affinity chromatography (IMAC). These modification-specific enrichment techniques are able to separate modified peptides from complex peptide mixtures, reducing sample complexity and improving the efficiency and reliability of the analysis.
| Enrichment methods developed for proteomics analysis of some common PTMs by LC-MS/MS |
|---|
| PTM types | Separation/enrichment techniques |
|---|
| Phosphorylation | IMAC, MOAC, SIMAC SCX, SAX, immunoaffinity purification, ERLIC |
| Acetylation | immunoaffinity purification, SCX, off-line fractionation using basic HPLC, and immunoaffinity purification RP-HPLC, HILIC |
| Ubiquitination | tagged-Ub, immunoaffinity purification, off-line fractionation using basic HPLC, and immunoaffinity purification |
| Glycosylation | lectin affinity chromatography, immunoaffinity purification, IMAC, MOAC, HILIC, ERLIC |
| Methylation | immunoaffinity purification,MBT domain affinity enrichment, SCX, HILIC |
| Sumoylation | tagged-SUMO, immunoaffinity purification |
The service offering at Creative Proteomics
Prior to the LC-MS/MS analysis, we apply a wide range of separation and enrichment methods to facilitate peptide separation in order to reduce sample complexity and expand PTM detection and coverage. Our peptide enrichment services take into account the specific requirements of our clients' projects and provide customized enrichment solutions that combine a variety of factors, including experimental scope, enrichment efficiency, ability to adapt to biological systems, and complexity of the analyte. We are dedicated to providing a perfect solution for the separation and enrichment of multiple modified peptides, facilitating the process of protein PTM identification, PTM quantification, and modified protein profiling in complex biological matrices and other complex mixtures. Specifically, our service consists of the following enrichment strategies:
Ion-exchange chromatography (IEC), including SAX and SCX, is a common method for protein and peptide separation. Here, our expert team is providing SAX and SCX chromatography for the fractionation of PTMs to facilitate the project process for our customers.
HILIC is based on the hydrophilic interaction between the analyte and the hydrophilic stationary phase. HILIC and electrostatic repulsion-hydrophilic interaction chromatography (ERLIC) are promising alternatives to ion-exchange chromatography for the pre-fractionation and enrichment of phosphopeptides and other modified peptides, such as glycopeptides and methylated peptides.
RPLC is based on the hydrophobic interactions of the protein/peptides with the RPC stationary phase for protein/peptide separation and enrichment. Due to its powerful resolving capability, recovery, and reproducibility, RPLC is the most prevalent separation technique in top-down proteomics.
IMAC utilizes trivalent metal ions immobilized on a stationary phase to selectively chelate negatively charged phosphate groups of phosphoproteins or phosphopeptides based on the affinity. This affinity purification technique has been commonly exploited to enrich phosphorylated proteins and peptides.
MOAC is a kind of affinity chromatography (AC) that is based on the affinity that the negatively charged phosphate groups toward metal oxides and is one of the most widely used methods for phosphopeptide enrichment. Compared to conventional phosphoprotein and phosphopeptide enrichment methods, MOAC exhibits higher sensitivity and selectivity. With our experienced experts and improved IMAC workflow, we are dedicated to meeting our customers' specific separation needs.
Immunoaffinity purification with immobilized antibodies is a well-established method for post-translationally modified peptide enrichment. Our antibody immunoaffinity precipitation service can help enrich a range of specific types of PTMs for our global customers, such as protein phosphorylation, acetylation, ubiquitination, and so on.
Lectin affinity chromatography (LAC) generally refers to enrichment with lectins and has been extensively used for the enrichment of glycopeptides. We have developed M-LAC for enriching and analyzing glycoproteins from various complex samples. Our services are flexible and customized, designed to provide the best fit for the diverse needs of our clients.
In MS-based PTM proteomics analysis, we recommend using a combination of separation and enrichment techniques to reduce sample complexity, improve selectivity, and increase the number of modified peptides identified. We insist on continuous optimization in our delivery process and service standards, which can guarantee an efficient service process and the accuracy and stability of the final delivered results. If you are interested in our services, do not hesitate to contact us. We will provide you with strong technical support to save your heart and effort.
References
- Huang, He, et al. "Quantitative proteomic analysis of histone modifications." Chemical reviews 115.6 (2015): 2376-2418.
- Brandi, Jessica, et al. "Advances in enrichment methods for mass spectrometry-based proteomics analysis of post-translational modifications." Journal of Chromatography A (2022): 463352.
Our products and services are for research use only.

 Fig. 1 Summary of the most common affinity enrichment strategies for PTMs analysis. (Brandi, Jessica, et al, 2022)
Fig. 1 Summary of the most common affinity enrichment strategies for PTMs analysis. (Brandi, Jessica, et al, 2022)