Disulfide bond is a special post-translational modification (PTM), a form of intra- or inter-chain cysteine linkage of proteins, which plays an important role in the correct folding of the higher structure of proteins and their maintenance of biological activity. With the increasing use of proteins in biological therapies, especially monoclonal antibodies with a high degree of disulfide bonding, it is emerging more and more important to detect and analysis disulfide bond and its variants in protein therapies. Therefore, as a critical quality attribute of proteins, disulfide bond must be monitored closely during the development of biotherapeutics. Creative Proteomics offers protein disulfide bond analysis solutions based on high-precision mass spectrometry (MS), which can determine the location of disulfide bonds by analyzing the sequence of peptides before and after reduction.
Functions of disulfide bonds
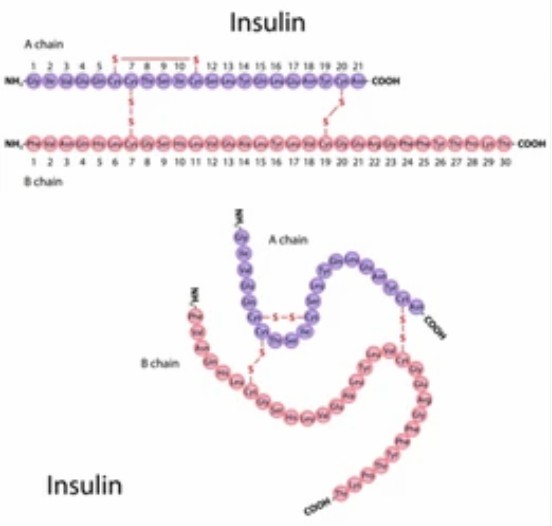
Formation of disulfide bonds is a common PTM and can be found in almost all species of viruses, prokaryotes and eukaryotes. As a structural building block, a disulfide bond covalently links two cysteine residues in the same protein or in different proteins to strengthen the correct conformation of a protein or protein complex, thus enabling the protein to maintain its normal folded form, form the correct isoform, and maintain a higher level of conformation to perform its normal biological functions. Moreover, the disulfide bond is reversible, so it can act as a molecular switch to regulate the activity of enzymes or transcription factors in response to the redox state of the environment. There are many secreted proteins riched in disulfide bonds, including growth factors, antibodies, cell surface receptors or transporters, and extracellular matrix proteins. Therefore, it is increasingly important to understand the biological functions of disulfide-containing proteins and their regulatory mechanisms, and in addition, the precise resolution of disulfide bond formation is an important factor to be examined in the production of protein products and biological drugs.
Protein disulfide bond analysis service
Our protein disulfide bond analysis service is based on high-precision mass spectrometry (MS). Our service generally includes sample preparation, liquid chromatography-MS/MS analysis, including choice of fragmentation techniques, and rapid disulfide bond analysis, providing a full range of analytical services. We can accept the minimum amount of protein required for testing, which can be as low as a few hundred ng for purified proteins or a few tens of μg for a mixture of hundreds of proteins. All you need to do is tell us the purpose of your experiment and send us your samples, and we will take care of all the follow-up of the project and provide information about the protein disulfide bonding sites, including:
- Localization analysis of disulfide bonds.
- Mapping all the disulfide bonds in a protein.
- Identification of disulfide bonded peptides.
- Consistency analysis of disulfide bonded peptides.
Sample requirements
- Acceptable samples: Protein extracts, cell samples, tissue samples, and microorganism samples.
- Sample shipping: Sufficient amount of dry ice for shipping, or consult our technical staff before sending samples.
- Before the formal experiment, we will always test the samples you provide.
Creative Proteomics is dedicated to achieving high precision and sensitivity in disulfide bond analysis at low sample sizes for researchers involved in the development of protein characterization methods. Notably, we specialize in targeting individuals with biotherapeutic profiles, specifically by disulfide bond mapping in antibodies, to provide optimal strategies for disulfide bond assignment of their bioproducts. Don't hesitate to contact us for more about our protein disulfide bond analysis service. We are looking forward to working with you on your next projects.
References
- Lakbub, Jude C., Joshua T. Shipman, and Heather Desaire. "Recent mass spectrometry-based techniques and considerations for disulfide bond characterization in proteins." Analytical and bioanalytical chemistry 410.10 (2018): 2467-2484.
- Landeta, Cristina, Dana Boyd, and Jon Beckwith. "Disulfide bond formation in prokaryotes." Nature microbiology 3.3 (2018): 270-280.
Our products and services are for research use only.