Protein prenylation is a C-terminal lipid modification and has been found to play an important role in
protein-membrane and protein-protein interactions, and signal transduction. As a professional PTM analysis service
provider, Creative Proteomics offers various post-translational modification (PTM) analysis
services to help our customers to accelerate research progress and obtain better results. Here, we also offer a
protein prenylation analysis service. With an advanced platform and strict workflow, we are able to analyze protein
prenylation in various complex samples to meet your requirements.
Protein prenylation
Protein prenylation, also called geranylgeranylation, is an irreversible PTM that refers to the process of attaching
two types of isoprenoid groups (a farnesyl or geranylgeranyl isoprenoid) to a C-terminal cysteine motif via farnesyl
transferase, geranylgeranyl transferase, and other cellular prenyltransferase enzymes. This modification enhances
the hydrophobicity and promotes membrane anchoring of Rho proteins, which is considered essential for proper
subcellular targeting, effector binding, GTP loading and activation. As an evolutionarily conserved modification,
prenylation has attracted extensive research interest for several reasons. It is reported that prenylated proteins
have been linked to diseases such as cancer, inflammation and premature aging. Therefore, enzymes that catalyze
reactions to generate mature prenylated proteins are being investigated as drug targets for related diseases. In
addition, prenylated proteins play an important role in plant developmental processes and stress response because
the incorporation of hydrophobic prenyl chains (mainly farnesyl or geranyl alcohol) makes hydrophilic proteins into
peripheral lipid membrane proteins.
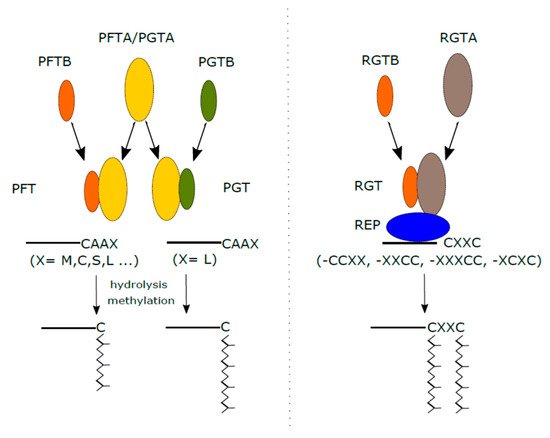 Fig. 1 Protein prenylation in plants. (Hála,
Michal, and Viktor Žárský. 2019)
Fig. 1 Protein prenylation in plants. (Hála,
Michal, and Viktor Žárský. 2019)
Proteomics analysis of prenylation
Prenylated proteins are well conserved across species, highlighting the biological and evolutionary importance of
this lipid modification regulation. Over the years, we have developed highly efficient MS-based proteomics
technology for system-wide identification and quantification of PTMs, including protein prenylation. Since metabolic
labeling has been widely used in the analysis of protein prenylation, our expert team uses metabolic labeling
methods in combination with enrichment. The obtained prenylated proteins will be identified by subsequent trypsin
digestion and LC-MS analysis. Based on a powerful and professional bioinformatics platform, we are capable of
helping our clients achieve efficient and rapid prenylated protein identification and quantification. Our service
consists of five major steps:
- Metabolic labeling in conjunction with enrichment.
- On-bead tryptic digestion.
- LC-MS/MS-based proteomics.
- MS data analysis.
- Bioinformatics analysis.
Major benefits of our service
- Cutting-edge technology and advanced platform.
- High-throughput facility and strict workflow.
- Powerful PTM analysis competence.
- Customized services from experienced experts.
The identification of intracellular prenylated proteins provides insight into the cellular processes and the etiology
of disease associated with prenylation, providing the basis for the development of new, necessary and highly
valuable related drug targets. Creative Proteomics continues to improve our resources and expand
our services, and is committed to providing high-quality services to accelerate our customer service projects. We
have assisted and witnessed the success of many of our partners. In order to get more details about our prenylation
analysis service, please contact us. We've got everything covered for your
needs.
References
- Wang, Mei, and Patrick J. Casey. "Protein prenylation: unique fats make their mark on biology."
Nature reviews Molecular cell biology 17.2 (2016): 110-122.
- Hála, Michal, and Viktor Žárský. "Protein prenylation in plant stress responses." Molecules
24.21 (2019): 3906.
Our products and services are for research use only.

 Fig. 1 Protein prenylation in plants. (Hála,
Michal, and Viktor Žárský. 2019)
Fig. 1 Protein prenylation in plants. (Hála,
Michal, and Viktor Žárský. 2019)