Immunoaffinity precipitation is an effective and powerful approach for purifying selective protein post-translational modifications (PTMs) for mass spectrometry (MS)-based proteomics analysis. Prior to MS analysis, Creative Proteomics offers antibody-based and enrichment strategies of post-translationally modified peptides that facilitate the study of PTMs in a wide variety of cells and tissues, as well as the investigation of various disease states. Specifically, our antibody immunoaffinity precipitation service can help enrich a range of specific types of PTMs for our global customers, such as protein phosphorylation, acetylation, ubiquitination, and so on.
Overview of antibody-based immunoaffinity precipitation
Immunoaffinity purification with immobilized antibodies is a well-established method for post-translationally modified peptide enrichment. Antibodies, including pan- (sequence-independent) antibodies and sequence specific anti-PTM antibodies, are valuable reagents for the separation and enrichment of PTMs. Pan antibodies recognize the modification, but not its surrounding sequence, which is used to assess the overall change in the protein PTMs of interest. While a sequence specific antibody can recognize the PTM moiety and its surrounding sequence and has been used to detect a specific PTM sites. Pan- and sequence-specific antibodies are produced similarly to protein antibodies, but with different antigen designs and antibody purification methods. Since the quality of PTM antibodies is critical for the effective separation of PTM peptides, the generated PTM antibodies of interest should be carefully examined so that they do not cross-react with other structurally similar PTMs.
Antibody-based immunoaffinity precipitation for modified peptide enrichment
To purify the modified peptide, the antibody is first coupled to a solid support. The beads are then incubated with a complex peptide mixture (extracted from cell lysates) at neutral pH to promote the binding of the modified peptides. Depending on the complexity of the antibody and peptide samples used, the incubation time can vary from 30 minutes to overnight. Finally, the enriched peptides are eluted using acidic buffer conditions and then used for MS analysis. Based on our state-of-the-art instruments and experienced experts, we offer an improved workflow for the separation and enrichment of modified peptides by immunoaffinity precipitation. Our service minimizes the loss of PTM peptides of interest and increases enrichment specificity, thus allowing the instrument to identify more peptides of interest and improving the identification and quantitative accuracy of MS-based methods.
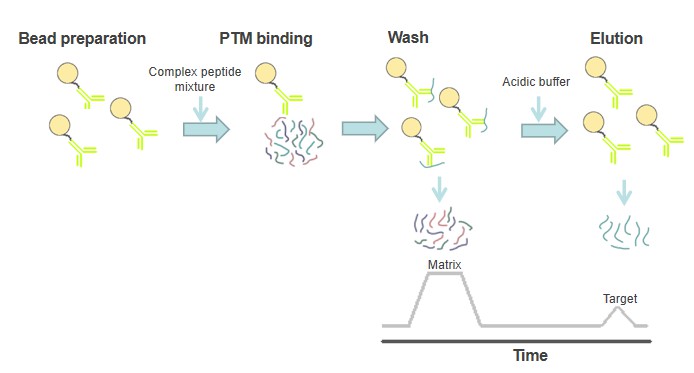 Fig. 1 Method overview for immunoaffinity purification.
Fig. 1 Method overview for immunoaffinity purification.
- Phosphopeptide enrichment
Using specific phosphoserine- and phosphothreonine-specific antibodies for separation of phosphoserine and phosphothreonine peptides. Antibody-based immunoaffinity precipitation for the enrichment of phosphopeptides is highly specific and efficient.
- Acetylated peptide enrichment-pan acetylation antibody-based affinity enrichment
Pan-acetyl lysine antibody-based affinity enrichment is the most commonly used method for the enrichment of acetylated peptides. Prior to immunoprecipitation with pan-acetyl lysine antibodies, we typically use trypsin to cleave large proteins into multiple small peptides as well as expose the acetyl-lysine to enhance affinity enrichment.
- Ubiquitinated peptide enrichment
Our ubiquitinated peptide enrichment service is primarily based on an anti-K-ε-GG antibody-based approach that specifically identifies lysine peptides containing ubiquitinated sites. Based on our robust workflow and experienced experts, we are able to greatly enhance the sensitivity of ubiquitination analysis, enhancing the comprehensive mapping scale of ubiquitination signals.
Major benefits of our service
- Professional and precise experimental design.
- Advanced and stable platform to provide high-quality data.
- Improved workflow with higher sensitivity and specificity.
- Customized enrichment strategies according to specific experimental objectives.
Related services
If you are interested in our services, do not hesitate to contact us. We will provide you with strong technical support to save your heart and effort.
References
- Wu, Chaochao, et al. "Contributions of immunoaffinity chromatography to deep proteome profiling of human biofluids." Journal of Chromatography B 1021 (2016): 57-68.
- Huang, He, et al. "Quantitative proteomic analysis of histone modifications." Chemical reviews 115.6 (2015): 2376-2418.
Our products and services are for research use only.


 Fig. 1 Method overview for immunoaffinity purification.
Fig. 1 Method overview for immunoaffinity purification.