Arginylation, a post-translational modification (PTM) involving the addition of arginine residues to proteins, plays a pivotal role in cellular functions and protein dynamics. This arginyltransferase-catalyzed enzymatic process has been identified as a critical regulatory mechanism affecting the localization, stability, and interaction networks of proteins. Arginylation analytical studies have far-reaching implications across various research areas, offering a multifaceted lens through which researchers can explore fundamental biological processes and address pressing questions in health and disease. As analytical techniques evolve, the depth of Creative Proteomics insights into arginylation's role in cellular regulation and pathology is poised to expand, unlocking new avenues for translational research and therapeutic innovation.
Analytical Methods for Arginylation
- Mass Spectrometry. Mass spectrometry (MS) stands out as a cornerstone in arginylation analysis. High-resolution MS allows for the identification and quantification of arginylated peptides, offering insights into the extent and site-specificity of this modification. Recent developments in tandem mass spectrometry (MS/MS) techniques have enhanced the reliability of detecting arginylation, even in low-abundance proteins.
- Antibody-Based Approaches. Immunoprecipitation using arginylation-specific antibodies has proven instrumental in isolating arginylated proteins. Coupled with Western blotting, this method facilitates the qualitative assessment of arginylation levels in complex biological samples, providing a complementary approach to mass spectrometry.
Applications of Arginylation Analysis
- Cancer Biology. Arginylation's role in cell cycle regulation and apoptosis places it at the forefront of cancer research. Analytical studies on arginylation can uncover novel biomarkers and therapeutic targets, fostering advancements in cancer diagnosis, prognosis, and treatment. Understanding the intricate regulatory networks involving arginylation may pave the way for targeted therapies that disrupt cancer progression.
- Neurodegenerative Disorders. The link between arginylation and neurodegenerative disorders, such as Alzheimer's and Parkinson's disease, presents a compelling avenue for research. Analytical investigations into the aberrant arginylation patterns in neuronal cells can deepen our understanding of the pathogenesis of these disorders.
- Cellular Stress Response. Arginylation is intricately connected to cellular stress responses, including responses to oxidative stress and heat shock. Analytical studies elucidating the dynamics of arginylation under different stress conditions can contribute to our understanding of cellular adaptation and survival mechanisms.
- Protein Quality Control and Degradation. Arginylation's interplay with other post-translational modifications, such as ubiquitination, underscores its involvement in protein quality control and degradation pathways. Analytical studies elucidating the cross-talk between arginylation and other degradation mechanisms can provide insights into cellular proteostasis. This knowledge is critical for understanding how cells maintain protein quality and respond to misfolded or damaged proteins.
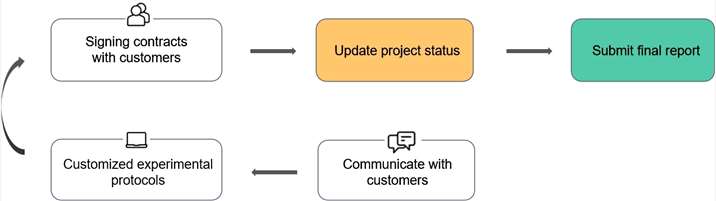
Our Service Workflow
Arginylation stands as a dynamic player in the intricate landscape of post-translational modifications. Through cutting-edge analytical methods and insightful applications, our researchers are unraveling the significance of arginylation in health and disease. As we delve deeper into the complexities of this modification, the potential for therapeutic interventions and targeted drug development becomes increasingly evident, opening new frontiers in the field of molecular biology.
Cysteine-Based Mimic of Arginylation Reproduces Neuroprotective Effects of the Authentic Post-Translational Modification on α-Synuclein
Journal: JACS
Published: 2023
Background
α-Synuclein (αS) is closely related to the pathogenesis of some neurodegenerative diseases such as Parkinson's disease. In functional studies of αS, arginylation modification as a post-translational modification has attracted the attention of scientists. Arginylation modification occurs on the glutamate side chain at positions 46 and 83 of αS. It has been shown that a decrease in the level of the arginylation modification leads to aggregation of αS in the mouse brain, producing neurotoxicity.
Chemical synthesis of sufficient amounts of arginine-modified αS for in vivo and in vitro studies is still difficult, so the authors took an alternative approach and considered the use of mimetics with the following properties: on the one hand, structurally similar to arginine-modified glutamate, and on the other hand, experimentally easy to synthesize. In this paper, the authors developed an arginine-modified mimic based on cysteine and demonstrated that it can produce functions similar to those of arginine modification, i.e., inhibit protein aggregation and exert neuroprotective effects.
Results
To obtain an arginylated mimic, the authors first synthesized a small molecule of bromoacetylarginine, synthesized mimic modification C (EArg) via nucleophilic substitution reaction of sulfhydryl groups, and introduced fluorescent motifs in conjunction with genetic coding techniques for subsequent characterization. Experimentally, the authors claim a doubling of protein synthesis yield using the mimic and simpler experimental steps (Figure 1).
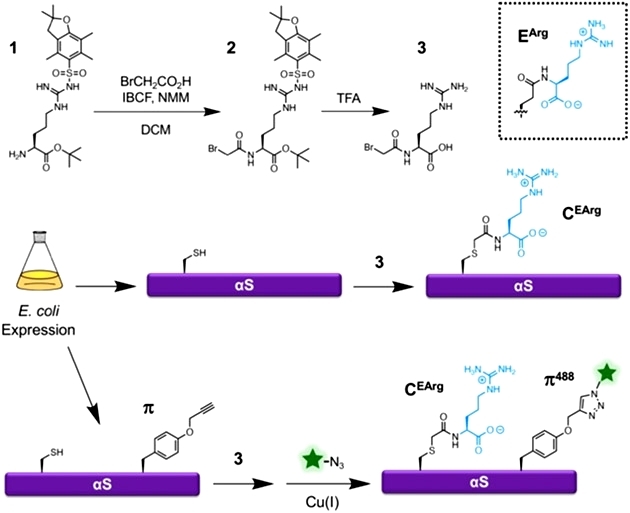 Figure 1
Figure 1
Since αS is a neuronal protein that regulates synaptic vesicle release, to test the functional impact of the mock modification on αS, the authors measured its lipid vesicle affinity by fluorescence correlation spectroscopy. The results showed that the C(EArg) modification had a similar effect on the lipid affinity of the protein as the true E(Arg) modification. To investigate whether the mimics could reduce protein aggregation, the authors analyzed the aggregation kinetic characteristics of αS at different modification levels at different sites. The results showed that the mimetic modifications at both sites slowed down protein aggregation to a certain extent; with about 35% inhibition achieved at a 5% mimetic modification level for both site 46 and site 83. Finally, the authors tested the aggregation seeding capability of the mock-modified αS protofibrils in cells. In neurons, both the mock modification and the true arginylation modification showed similar and significant inhibition of the aggregation seeding activity of the protofibers (Figure 2).
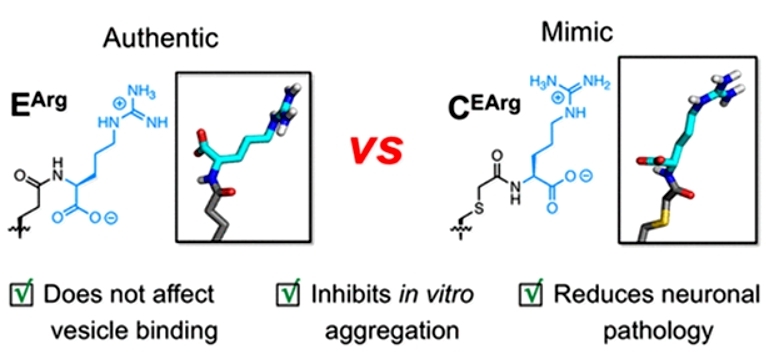 Figure 2
Figure 2
Conclusion
The authors have invented an arginylated post-translational modification mimic that improves the synthetic efficiency of experiments and accelerates functional studies of post-translational modifications of proteins while essentially not affecting protein function.
Reference
- Pan B, Shimogawa M, Zhao J, et al,. Cysteine-Based Mimic of Arginylation Reproduces Neuroprotective Effects of the Authentic Post-Translational Modification on α-Synuclein. J Am Chem Soc. 2022 May 4;144(17):7911-7918.


 Figure 1
Figure 1 Figure 2
Figure 2