Palmitoylation is a fatty acylation of cysteine residues undergoing reversible lipid modifications that can modulate
target proteins in a variety of ways, such as regulating protein transport, stability, and cellular localization,
involving many biological processes that are relevant to the development of many diseases. The identification and
analysis of palmitoylated modified proteomes, including modification sites, is of great importance. To meet the need
for palmitoylation protein research and identification of palmitoylation sites in multiple samples, Creative
Proteomics offers a one-stop, customized protein palmitoylation analysis service.
Protein palmitoylation
Protein palmitoylation (S-acylation) is a thioester linkage of a lipid to a protein cysteine residue, of which
16-carbon fatty acid palmitate is the most common type. As one of the only known reversible lipid modifications of
proteins, palmitoylation is unique among other lipid modifications, acting as a regulatory PTM. By increasing
hydrophobicity at specific points in the protein structure and thus allowing protein binding/interaction with the
membrane, such protein modifications can regulate protein cell surface expression, transport, and
compartmentalization. In addition to soluble proteins associated with membranes via palmitoylation (e.g. Ras),
integral membrane proteins are also widely modified by palmitoylation, including modulating the topology/structure
of integral membrane proteins as well as regulating the accessibility of protein domains to enzymes that mediate
other regulatory PTMs.
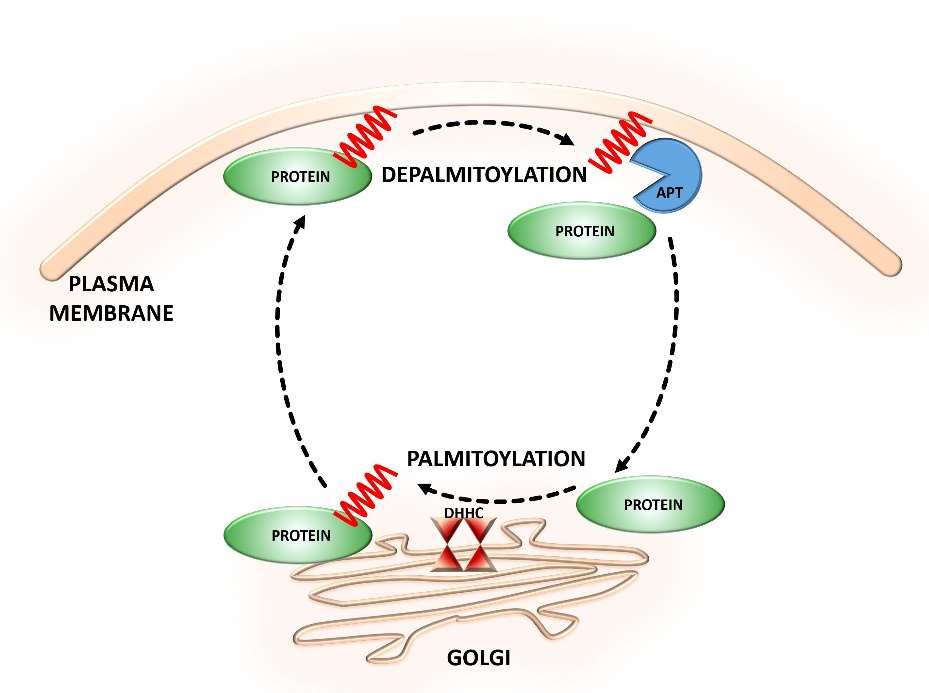 Fig. 1 Dynamic S-palmitoylation of proteins.
(Zaręba-Kozioł, Monika, et al., 2018)
Fig. 1 Dynamic S-palmitoylation of proteins.
(Zaręba-Kozioł, Monika, et al., 2018)
Proteomics analysis of palmitoylation
A variety of different methods have been developed to investigate protein palmitoylation. Creative
Proteomics provides fast and efficient protein palmitoylation identification and analysis services with
a professional and high-throughput liquid chromatography-tandem mass spectrometry (LC-MS/MS) platform. To perform a
more accurate palmitoylation analysis of proteins, we used a combination of labeling methods and then compared
palmitoylated proteins identified from each method with each other by MS. Our integration and optimization services
enable the enrichment and reliable identification of many palmitoylated protein and palmitoylation sites.
Specifically, our services include protein extraction and trypsin digestion, optimized enrichment of palmitoylated
proteins, LC-MS/MS analysis, database searching and analysis, and customized bioinformatics analysis.
Service features
- Significant enrichment selectivity and sensitivity, enabling global annotation of protein palmitoylation in
complex biological samples.
- Get more information about peptides and identify and analyze specific palmitoylation sites.
- Large-scale identification of the enriched palmitoylated peptides by powerful MS analysis.
- A research team with extensive experience in PTMs.
- Provide a variety of bioinformatics analyses.
Bioinformatics analysis
- MS data quality control
- Palmitoylated protein and palmitoylation site identification
- Quality assessment of identification results
- Functional annotation of palmitoylated proteins
- Motif analysis of phosphorylated peptides
Workflow of protein palmitoylation analysis

Sample requirements
- Acceptable samples: Protein extracts, cell samples, tissue samples, and microorganism samples.
- Sample shipping: Sufficient amount of dry ice for shipping, or consult our technical staff before sending
samples.
- Before the formal experiment, we will always test the samples you provide.
Protein PTMs are one of the major biological pathways regulating many biological processes and diseases. Our MS-based
proteomic analysis of protein palmitoylation enables the identification and analysis of palmitoylated proteins in a
wide range of eukaryotic and prokaryotic samples. Please feel free to contact
us for more information. We are always ready to assist you!
References
- Zaręba-Kozioł, Monika, et al. "Insights into protein S-palmitoylation in synaptic plasticity and
neurological disorders: potential and limitations of methods for detection and analysis." Frontiers in
Molecular Neuroscience 11 (2018): 175.
- Collins, Mark O., Keith T. Woodley, and Jyoti S. Choudhary. "Global, site-specific analysis of neuronal
protein S-acylation." Scientific reports 7.1 (2017): 1-14.
Our products and services are for research use only.

 Fig. 1 Dynamic S-palmitoylation of proteins.
(Zaręba-Kozioł, Monika, et al., 2018)
Fig. 1 Dynamic S-palmitoylation of proteins.
(Zaręba-Kozioł, Monika, et al., 2018)