Post-translational modifications (PTMs) dramatically enhance the diversity, activity, and capabilities of the protein by modulating intra- and intermolecular interactions. In the past, researchers believed that PTMs occurred mainly on amino acid side chains, and that polypeptide backbone was generally considered to be inert. As protein PTMs have been intensively studied, it has been found that backbone PTMs can control the structure and function of proteins like side chain modifications and achieve these functions through unique mechanisms. Creative Proteomics is a leading customized service provider in PTM proteomics analysis and is dedicated to the comprehensive and reliable identification, quantification, and prediction of protein PTMs. We now offer protein backbone PTM analysis services, which are mainly based on protein mass spectrometry (MS) analysis methods combined with enrichment strategies.
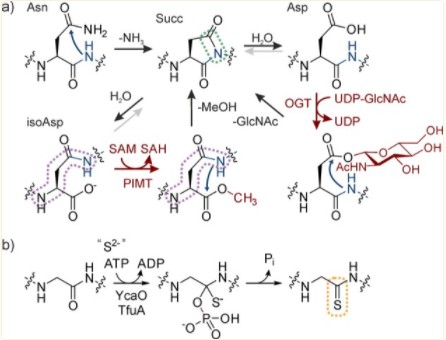 Fig. 1 Installation of backbone modifications by spontaneous and enzymatic pathways. (Müller, Manuel M., 2018)
Fig. 1 Installation of backbone modifications by spontaneous and enzymatic pathways. (Müller, Manuel M., 2018)
Overview of protein backbone modifications
By repeatedly forming amide bonds within the ribosome, proteins are assembled from a typical set of 20 α-L-amino acids. Following their biosynthesis, these proteins are further tuned and undergo new chemical reactions through covalent modifications. Moreover, PTMs act as important regulators or even as on/off switches, dynamically or irreversibly controlling the localization and activity of most natural proteins. Traditionally, PTM was thought to occur only on amino acid side chains, a paradigm that is changing with the discovery of chemically and functionally diverse alterations in the protein backbone. Indeed, backbone modifications are ubiquitous in peptide natural products and they include chemically conservative modifications and substantial alterations to the backbone. There are some major effects of backbone PTMs on proteins, including,
- Impart new properties to proteins.
- Constitutively enhance protein stability and activity.
- Regulate protein activity.
Protein backbone PTM identification and analysis

Structural biology represents an important toolkit for the identification of backbone PTMs, which was initially revealed by X-ray crystallography studies. However, structural biology has limitations in terms of throughput and bias in identifying backbone PTMs. To help our customers systematically explore the role of backbone PTMs in biology, we offer MS-based analysis methods combined with specific enrichment strategies. And our customers are working on other technologies to enrich and identify more backbone PTMs.
Workflow of our service
- Protein extraction and digestion.
- Peptide enrichment.
- High-end MS-based analysis.
- Systems-level analysis of backbone-modified proteins.
Advantages of our service
- Powerful mass spectrometric analysis.
- Rich experience in PTM crosstalk identification and analysis.
- Advanced platform and professional experts to provide high-quality data.
- Responsive project management with considered and comprehensive services.
Our PTM proteomics analysis platform has a team of experts and state-of-the-art equipment to help you navigate successful research projects through strong workflow, robust analysis, and tailored services. For more information on our services, please use our contact form. Our professional staff looks forward to working with you on your next project.
Reference
- Müller, Manuel M. "Post-translational modifications of protein backbones: unique functions, mechanisms, and challenges." Biochemistry 57.2 (2018): 177-185.
Our products and services are for research use only.

 Fig. 1 Installation of backbone modifications by spontaneous and enzymatic pathways. (Müller, Manuel M., 2018)
Fig. 1 Installation of backbone modifications by spontaneous and enzymatic pathways. (Müller, Manuel M., 2018)