Citrullination, a post-translational modification (PTM), involves the conversion of arginine residues in proteins to citrulline. This enzymatic process is catalyzed by peptidylarginine deiminases (PADs), a family of calcium-dependent enzymes. While originally identified in the context of autoimmune diseases such as rheumatoid arthritis, citrullination has emerged as a crucial cellular mechanism with implications beyond autoimmunity.
Role of Citrullination
- Autoimmunity and Beyond. Citrullination's pivotal role in autoimmune diseases stems from its association with the generation of anti-citrullinated protein antibodies (ACPA). These antibodies target citrullinated proteins, contributing to the pathology of diseases like rheumatoid arthritis. However, recent research has broadened our understanding, revealing citrullination's involvement in other physiological processes.
- Epigenetic Regulation. Beyond autoimmunity, citrullination plays a role in epigenetic regulation. The modification affects chromatin structure, impacting gene expression. PAD enzymes, known for their involvement in citrullination, have been linked to the modulation of gene transcription through histone citrullination. This expands the significance of citrullination into the realm of gene regulation.
Analytical Methods of Citrullination
- Mass Spectrometry. Analyzing citrullination necessitates cutting-edge techniques, with mass spectrometry leading the pack. High-resolution mass spectrometry enables the identification of citrullinated peptides, providing insights into the scope and dynamics of this modification.
- Antibody-Based Approaches. Immunodetection methods, particularly Western blotting and immunohistochemistry using anti-citrulline antibodies, remain invaluable tools for assessing citrullination. These approaches offer specificity and sensitivity in detecting citrullinated proteins in complex biological samples.
- Biochemical Assays. Enzymatic assays, employing recombinant PAD enzymes, enable the quantification of citrullination levels. These assays facilitate both in vitro studies and the assessment of endogenous citrullination in biological samples.
Applications of Citrullination Analysis
- Disease Biomarkers. The identification of citrullinated proteins as biomarkers holds promise for early disease diagnosis and prognosis. In rheumatoid arthritis, detecting ACPA has become a diagnostic hallmark, highlighting the clinical relevance of citrullination in disease management.
- Drug Development. Understanding citrullination's role in diseases opens avenues for therapeutic interventions. Small molecules targeting PAD enzymes are under investigation for their potential in modulating citrullination, offering new prospects for drug development in autoimmune and other citrullination-associated disorders.
- Epigenetic Modulation. Given citrullination's involvement in epigenetic regulation, manipulating this modification may offer strategies for controlling gene expression. This has implications not only in disease treatment but also in the broader context of synthetic biology and genetic engineering.
Important Aspects in Citrullination Analysis
- Site-Specific Analysis. The site-specific identification of citrullination sites within proteins is critical for understanding its functional implications. Advances in mass spectrometry-based proteomics have enabled precise mapping of citrullination sites, providing nuanced insights into protein regulation.
- Quantitative Assessment. Quantifying citrullination levels under different conditions is essential for unraveling its dynamic nature. Integrating quantitative mass spectrometry approaches with biochemical assays enhances our ability to quantify citrullination changes in response to various stimuli or disease states.
- Cross-Talk with Other PTMs. Exploring the cross-talk between citrullination and other PTMs unveils the complexity of cellular signaling networks. Interactions with acetylation, methylation, and phosphorylation highlight the interconnectedness of different regulatory mechanisms, providing a holistic view of protein regulation.
Our Service Advantages
Citrullination, once confined to the realm of autoimmune diseases, has evolved into a multifaceted player in cellular processes. From epigenetic regulation to disease biomarkers and drug development, the implications of citrullination are far-reaching. Creative Proteomics is dedicated to protein research, we utilize various analytical methods to help researches understand the citrullination's intricacies.
Citrullinated fibrinogen-SAAs complex causes vascular metastagenesis
Journal: Nat Commun
Published: 2023
Background
Cancer metastasis refers to the process by which cancerous cells spread from the primary tumor site into other regions of the body, and despite the great strides scientists are making in the field of cancer research today, the drivers that drive the selective spread of cancer to specific regions of the body are still largely unknown to researchers. In this article, researchers have identified one of these potential molecular mechanisms. By elucidating that post-translational citrullination of fibrinogen creates a metastatic habitat at susceptible sites, the researchers may reveal the possibility of detecting metastatic predictive sites, where lung endothelial cells mediate the citrullination of fibrinogen, which alters its conformation, surface charge, and binding properties associated with serum amyloid A or SAAs. SAAs, thus enabling them to become host tissue-derived metastatic pathogens.
Results
Citrullination is the process by which arginine in proteins is converted to citrulline; in this study, the researchers focused on specific modifications that occur in the protein fibrinogen, which contains one or more carbohydrate chains and plays a key role in the blood clotting process. To further elucidate the mechanisms of metastasis in lung cancer, after establishing a direct correlation between dispersed fibrinogen deposits in lung blood vessels and an increased likelihood of cancer metastasis, the researchers decided to scrutinize the biochemical modifications that occur after fibrinogen is synthesized (i.e., translated) in vivo, starting with dissected lung tissue samples obtained from individuals with and without cancer.
When stained with the dye, there was a significant increase in fibrinogen-positive areas in the lung tissue of cancer patients compared to those who were cancer-free at the time of death, and subsequent analyses showed that the expression of the serum amyloid A gene (SAA, a known pre-metastatic gene in the mouse organism) was five times higher in the cancer patients than in the bodies of individuals who did not have cancer. Based on this, the researchers then developed mice with humanized SAAs, i.e., rodents genetically programmed to produce a compatible version of the human SAA protein, and, unsurprisingly, metastasis of lung tumors was more severe in mice with humanized SAA clusters compared to mice with the SAA cluster gene blocked. Next, the researchers investigated the interaction between SAA3 proteins and fibrinogen in the lungs of tumor-bearing mice. During the course of their experiments, the researchers found that SAAs mediate the citrullination of fibrinogen and convert the standard amino acid arginine to the non-standard citrulline(Figure 1).
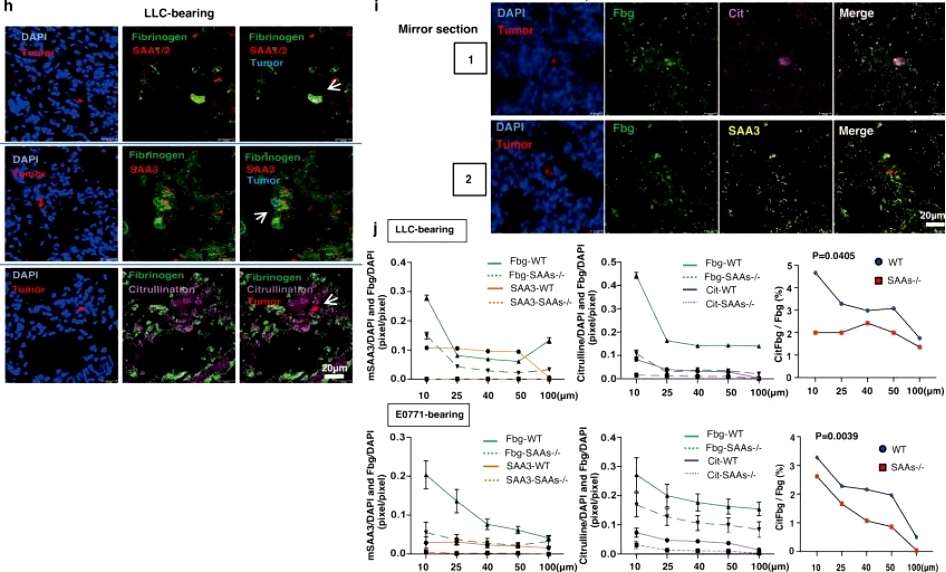 (Figure 1)
(Figure 1)
Further experimental results showed that SAAs-mediated citrullination of fibrinogen leads to tumor metastasis in the lungs in a mouse model, and finally, a protein complex containing citrullinated fibrinogen and SAAs (SAAs-CitFbg) is apparently recruiting cancer cells and can promote metastasis in lung tumors in a mouse model, Based on these results, the current researchers successfully designed a novel CitFbg peptide to combat cancer metastasis in a mouse model. Researcher Sachie Hiratsuka added that a CitFbg peptide may be able to act as a competitive inhibitor to block the homing process of metastatic cells to the SAAs-CitFbg sites, and that potential metastatic sites in patients' lungs could be clearly visualized by the CitFbg-specific antibody developed by the researchers(Figure 2).
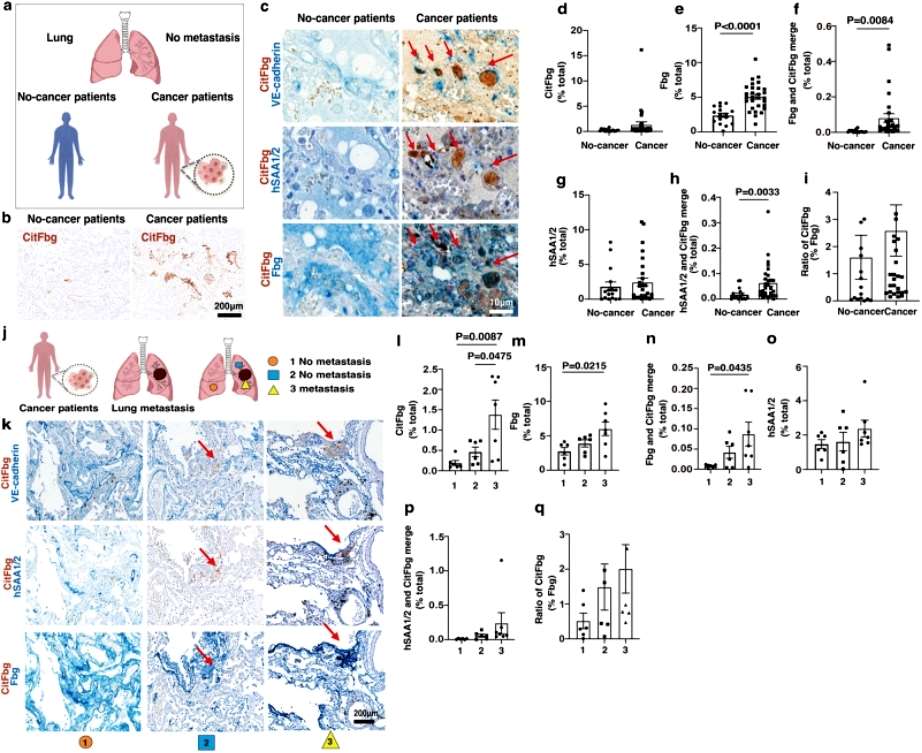 Figure 2
Figure 2
Conclusion
The results of this paper suggest that CitFbg deposition may be associated with an increased risk of metastasis in cancer patients and that citrullinated peptides may be effective in counteracting this in mouse models. The prediction of cancer metastasis may provide researchers with an opportunity to intervene early to mitigate the progression of cancer and the life-threatening risks associated with it, and although more clinical trials are needed, this study may pave the way for the development of novel anti-metastatic drugs in the future. The findings also add significant value to cancer research and may have an impact on the prevention and treatment of cancer with herbal medicines. In summary, the results of this paper suggest that the deposition of CitFbg may indicate the risk of metastasis in cancer patients and that citrullinated peptides may also be expected to serve as a specific class of cancer metastatic inhibitors.
Reference
- Han Y, Tomita T, Kato M, et al,. Citrullinated fibrinogen-SAAs complex causes vascular metastagenesis. Nat Commun. 2023 Aug 24;14(1):4960.


 (Figure 1)
(Figure 1) Figure 2
Figure 2