Protein lysine lactylation is a novel post-translational modification (PTM) widely found in fungal and mammalian
(e.g. human and mouse) cells that directly stimulates gene transcription and significantly affects downstream gene
expression and DNA replication. Understanding the role and regulatory mechanisms of lysine lactylation in
physiological and pathological processes that are highly dependent on glycolysis and lactate is essential.
Creative Proteomics is committed to providing customized and highly effective protein lactylation
analysis services and solutions to meet the specific needs of their projects.
Protein lactylation
Lactate is the end product of glycolysis and has long been recognized as a waste product that causes muscle fatigue
and potential tissue damage. As lactate research continues to advance, it plays an important role not only as an
intermediate in cellular metabolism but also as a signaling molecule for cellular communication. In general, protein
lactylation is a novel PTM that has been found to modify lysine residues on histones in response to a range of
cellular stresses (e.g. bacterial infection and hypoxia). The discovery of histone modification not only provides
the molecular basis for lactate regulation of gene expression but also greatly expands the biological functions of
lactate metabolism. In addition, it is reported that lysine lactylation is associated with a range of human
diseases, including ischemic heart and brain diseases, bacterial infection, tumor development, and so on.
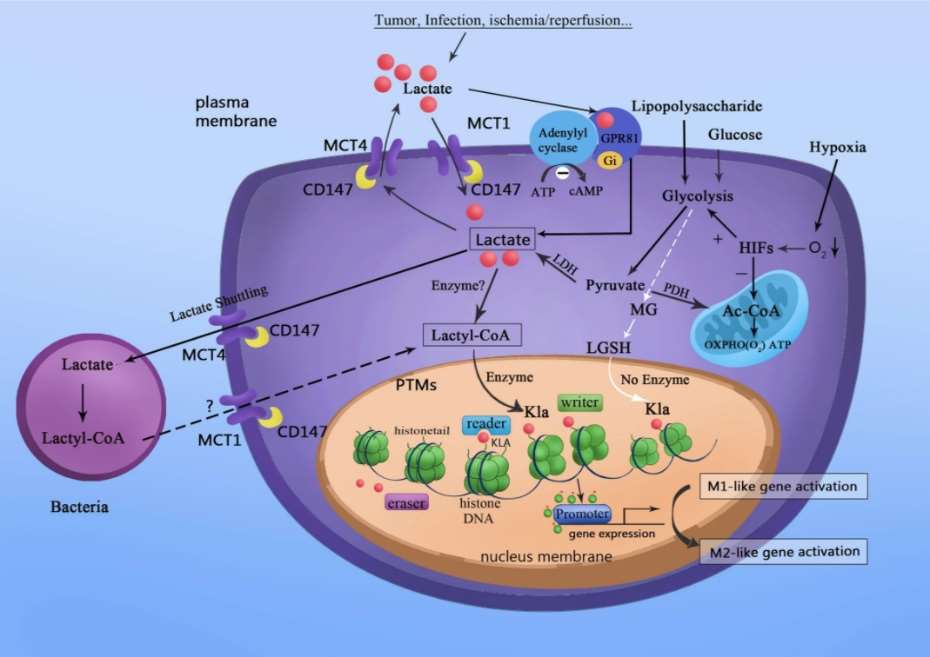 Fig.
1 Lactate acts as a signaling molecule to stimulate gene transcription via histone lysine lactylation in M1
macrophages. (Xin, Qi, et al., 2022)
Fig.
1 Lactate acts as a signaling molecule to stimulate gene transcription via histone lysine lactylation in M1
macrophages. (Xin, Qi, et al., 2022)
Proteomics analysis of lactylation
We have developed highly efficient mass spectrometry (MS) -based proteomics technology for system-wide identification
and quantification of PTM substrates and mapping their sites. As for lysine lactylation analysis, we combine
anti-lactate antibody-based immunoprecipitation with liquid chromatography-tandem mass spectrometry (LC-MS/MS)
techniques. Our expert team focuses on using a range of enzymatic and chemical cleavage methods, as well as
extensive fractionation, to maximize proteomic sequence coverage. In addition, our bioinformatics platform can
contribute to the understanding of the functional relevance of protein lactylation in cellular physiology. Our
services specifically include:
-Test the samples you provide before the official experiment.
-Protein extraction and digestion.
-Affinity enrichment of lysine lactylated peptides.
- LC-MS/MS analysis
- Bioinformatics analysis
-MS data quality control.
-Lysine lactylated protein and site identification and analysis.
-Lactylated site pattern analysis.
-Lactylated protein cellular localization and functional enrichment analysis.
-Protein-protein interaction (PPI) network of lactylated proteins.
Why choose us?
- Highly specific pan-antibodies.
- High-resolution, high-sensitivity MS.
- Experienced PTM research team.
Workflow of lysine lactylation analysis service

Applications of our service
- Lactylation in gene transcription.
- Lactylation in immune cells.
- Drugs targeting histone lactylation.
- In-depth study of human diseases and their processes.
- Discover a broader landscape of lactylation beyond histones.
Contact us today to learn more about our lysine lactylation analysis services.
References
- Hagihara, Hideo, et al. "Protein lactylation induced by neural excitation." Cell
Reports 37.2 (2021): 109820.
- Xin, Qi, et al. "Lactylation: a passing fad or the future of posttranslational modification."
Inflammation (2022): 1-11.
- Zhang, Di, et al. "Metabolic regulation of gene expression by histone lactylation."
Nature 574.7779 (2019): 575-580.
Our products and services are for research use only.

 Fig.
1 Lactate acts as a signaling molecule to stimulate gene transcription via histone lysine lactylation in M1
macrophages. (Xin, Qi, et al., 2022)
Fig.
1 Lactate acts as a signaling molecule to stimulate gene transcription via histone lysine lactylation in M1
macrophages. (Xin, Qi, et al., 2022)