The analysis of glycosylation sites in glycoproteins is important for the study of protein structure and function. As a leading service provider in the field of protein post-translational modification (PTM) analysis, Creative Proteomics provides a comprehensive glycosylation characterization service using mass spectrometry (MS)-based methods. Based on our excellent experts and advanced platform, we can help customers to solve the following aspects: whether glycosylation occurs, where glycosylation occurs, what is the type of glycosylation, the composition and sequence of various sugars in the glycan, etc.
Protein glycosylation
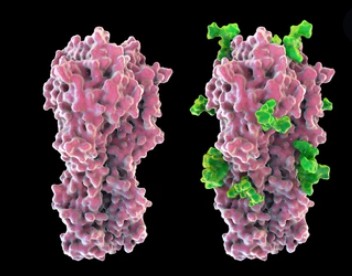
Protein glycosylation is the complex, multistep process in which carbohydrate (sugar) molecules react with protein amino acid side chain reactive groups catalyzed by glycosyltransferase (GTs) to generate a glycosidic linkage, thereby attaching the glycans to the protein. According to the type of chemical bonds in which glycosylation occurs, protein glycosylation is mainly classified as N-glycosylation and O-glycosylation. Glycosylation regulates the localization, function, activity, stability, and diversity of proteins in tissues and cells, and is involved in various important life activities including cell recognition, differentiation, development, signaling, and immune response. Aberrant protein glycosylation events have been found in a variety of diseases such as tumors, neurodegenerative diseases, cardiovascular diseases, metabolic diseases, immune diseases, and infectious diseases. In addition, as the most complex PTM, glycosylation has an impact on stability, solubility, higher-order structure, immunogenicity, and PK/PD of the protein. Therefore, glycosylation is considered a critical quality attribute (CQA) and accurate and reliable measurement and control of it are essential in therapeutic protein products.
Glycosylation site identification service
With the increasing resolution of MS and the continuous development of bioinformatics analysis, MS has been widely used in glycosylation analysis. Analysis of intact glycoproteins provides additional information on the overall glycoform distribution and the relative abundance of the corresponding proteoforms for comprehensive characterization. Our expert team uses next-generation combinatorial MS under different fragmentation regimes to resolve the intact glycopeptides of complex glycoproteins, and then interpret the raw MS data using mainstream bioinformatics analysis software, which enables the identification of N-glycosylation sites and O-glycosylation sites, and the analysis of the composition of the glycan chains carried by the corresponding sites.
- Identification of N-glycosylation sites in glycoproteins.
N-glycosylation involves the attachment of glycans to asparagine or arginine residues.
- Identification of O-glycosylation sites in glycoproteins.
O-glycosylation involves the attachment of glycans to serine, threonine, tyrosine, hydroxyproline, or hydroxylysine (HyK) residues.
Workflow of glycosylation site identification
- Selection of a suitable protease based on the amino acid sequence of the target protein.
- Performing enzymatic digestion.
- Glycopeptide enrichment.
- Liquid chromatography-mass spectrometry ((LC-MS) analysis.
- In terms of data analysis, the current international mainstream glycosylation data analysis software is used for data retrieval to effectively remove false positive results and improve the reliability of identification results.
Advantages of our service
- Identify glycosylation sites more accurately.
- Fast and flexible analytical capabilities.
- Excellent sensitivity and high-quality MS/MS data.
- Powerful and intuitive data analysis platform.
If you have any questions, or comments, or would like a free quote? Do not hesitate to contact us.
Reference
- Stavenhagen, Kathrin, et al. "Site-specific N-and O-glycosylation analysis of atacicept." MAbs. Vol. 11. No. 6. Taylor & Francis, 2019.
Our products and services are for research use only.