Creative Proteomics is a leading provider of custom protein post-translational modifications (PTMs). We are dedicated to providing a series of protein PTM analysis services, including protein crotonylation analysis. We can help our global customers to identify, analyze, and annotate protein crotonylation in a variety of complex biological samples. Lysine crotonylation is a newly discovered acylation modification and can occur in histones and non-histone proteins.
Protein crotonylation
Protein crotonylation occurs mainly on the ε-amino group of lysine in histones. Since the first discovery of lysine crotonylation in histones of mouse sperm and human cell lines, lysine crotonylation has attracted the attention of researchers. To date, there are thousands of crotonylation sites have been identified in proteins. Lysine crotonylation has been confirmed in animals, plants, and various microorganisms (e.g. bacteria and fungi). Crotonylation is a modification closely related to acetylation (an acetylation modification has been well characterized), and it has been reported that histone crotonylation not only has a similar structure but also shares the same set of enzyme systems as histone acetylation. Crotonylation has been disclosed to be involved in chromatin reorganization, nucleotide metabolism, RNA processing, protein activity and localization regulation, and other physiological processes. In addition, crotonylation has been linked to a myriad of diseases such as depression, HIV latency, and cancer.
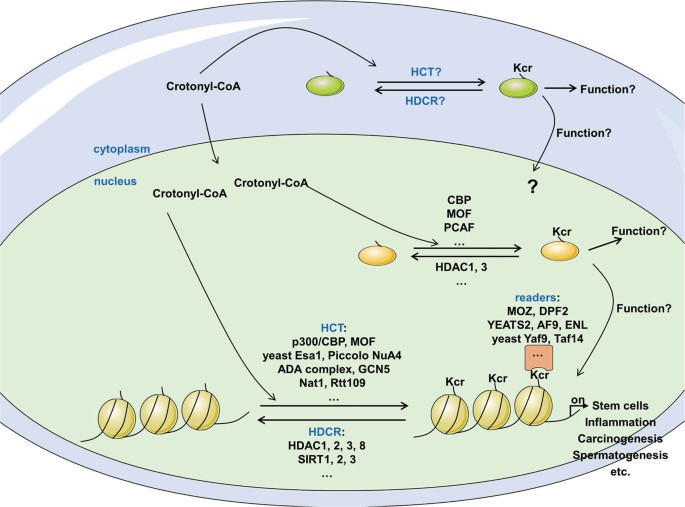 Fig. 1 The modulation of protein crotonylation. (Jiang, Gaoyue, et al., 2021)
Fig. 1 The modulation of protein crotonylation. (Jiang, Gaoyue, et al., 2021)
Proteomics analysis of crotonylation
Lysine crotonylation demonstrates a crucial role in a wide range of biological processes and may be critically implicated in the pathogenesis of diseases. We are dedicated to helping our clients investigate the role of lysine crotonylation in diseases and the underlying mechanism. Our PTM analysis platform is based on mainstream peptide enrichment strategies and high-resolution mass spectrometry (MS). According to the specific PTM, our experienced experts use methods with high specificity and efficient enrichment. Our protein crotonylation analysis service allows the identification and quantification of thousands of lysine crotonylation sites, enabling the whole-proteome characterization of lysine crotonylation. Specifically, our services include the following lists:
- Protein extraction and trypsin digestion.
- Tandem mass tag/isobaric tags for relative and absolute quantitation (TMT/iTRAQ) labeling.
- High-performance liquid chromatography (HPLC) fractionation.
- Protein crotonylation enrichment with affinity enrichment strategies.
- Liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis.
- MS database analysis.
- Bioinformatics analysis.
-MS data quality control.
-Lysine crotonylation subcellular localization prediction.
-Functional classification of lysine crotonylation in GO and lysine crotonylation subcellular localization.
-Functional enrichment of lysine crotonylation in GO, KEGG, and protein domain.
-Cluster analyses in GO, KEGG, and protein domain.
-Protein-protein interaction network of the crotonylated proteins.
-Analysis of crotonylation site motif.
Benefits of our service
- A professional and high-throughput LC-MS/MS platform.
- Significant enrichment selectivity and sensitivity.
- Get more information about peptides and identify and analyze specific crotonylation sites.
- Customized bioinformatics services from experienced bioinformatics experts.
Sample requirements
- Acceptable samples: Protein extracts, cell samples, tissue samples, and microorganism samples.
- Sample shipping: Sufficient amount of dry ice for shipping, or consult our technical staff before sending samples.
- Before the formal experiment, we will always test the samples you provide.
Crotonylated proteins have been reported to play important roles in the regulation of various biological processes and the pathogenesis of different diseases. If you have questions about our services, please don't hesitate to contact us for more information. We will arrange for professional staff to contact you as soon as possible!
References
- Jiang, Gaoyue, et al. "Protein lysine crotonylation: past, present, perspective." Cell death & disease 12.7 (2021): 1-11.
- Wang, Shuo, et al. "The Function and related Diseases of Protein Crotonylation." International Journal of Biological Sciences 17.13 (2021): 3441.
Our products and services are for research use only.

 Fig. 1 The modulation of protein crotonylation. (Jiang, Gaoyue, et al., 2021)
Fig. 1 The modulation of protein crotonylation. (Jiang, Gaoyue, et al., 2021)