Due to the heterogeneity and scarcity of the modified peptides, as well as dynamic modification processes in vivo, the detection of PTM usually requires enrichment strategies prior to mass spectrometry (MS) analysis. In addition to immobilized metal affinity chromatography (IMAC), Creative Proteomics also provides metal oxide affinity chromatography (MOAC) as an alternative method of phosphopeptide enrichment. Compared to conventional phosphoprotein and phosphopeptide enrichment methods, MOAC exhibits higher sensitivity and selectivity. In PTM analysis, we recommend using a combination of separation and enrichment techniques to reduce sample complexity, improve selectivity, and increase the number of modified peptides identified. With our experienced experts and improved IMAC workflow, we are dedicated to meeting our customers' specific separation needs.
Metal oxide affinity chromatography (MOAC) for phosphopeptide enrichment
MOAC is a kind of affinity chromatography (AC) that is based on the affinity that the negatively charged phosphate groups toward metal oxides and is one of the most widely used methods for phosphopeptide enrichment. MOAC is shown to be more tolerant to low pH and the presence of detergents than IMAC (another affinity purification technique widely used for phosphopeptide enrichment). There are several metal oxides (MOs) utilized for this technique, including TiO2, ZrO2, and Nb2O5. Of these, TiO2 is likely to be the most commonly used MOAC substrate with the strengths of high enrichment efficiency and high specificity. Since different MOs have different affinities and specificities for phosphopeptides, multiple MOs can be combined for enrichment to maximize the recovery of phosphopeptides.
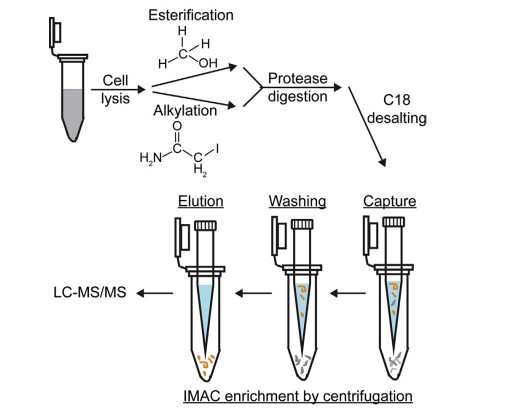 Fig. 1 Overview of phosphopeptide enrichment using immobilized metal affinity chromatography (IMAC). (Chang, Y-Y., H. Li, and H. Sun, 2017)
Fig. 1 Overview of phosphopeptide enrichment using immobilized metal affinity chromatography (IMAC). (Chang, Y-Y., H. Li, and H. Sun, 2017)
The service offering at Creative Proteomics
To enable deeper phosphorylation identification and quantitative, we offer a titanium dioxide metal oxide affinity chromatography (TiO2-MOAC) method for phosphopeptide enrichment. In a typical TiO2-based MOAC process, the sample is mixed with an acidic buffer to protonate the acidic residues of the non-phosphorylated peptides and prevent them from binding to positively charged TiO2. After the washing step is completed, the phosphopeptides are eluted from the column under alkaline conditions. Our team of experts has optimized the protocols and buffer formulations in a TiO2-MOAC based workflow dedicated to greatly improving the yield and specificity of phosphopeptides for complex protein digestion. Our capability includes cell culture and treatment, sample preparation, phosphopeptide enrichment and fractionation. To ensure a more complete enrichment of the phosphopeptides, our professional experts have explored the use of multiple enrichment strategies. We also offer sequential elution from IMAC (SIMAC), which combines IMAC and TiO2 enrichment strategies to enrich phosphopeptide in a consecutive manner, which can greatly facilitate the identification of more phosphorylation sites.
Major benefits of our service
- Robust protocol, simple procedure, and high selectivity for phosphopeptide enrichment.
- Improved workflow with higher sensitivity and reduced analysis time.
- Multiple methods with high flexibility.
- Experienced protein enrichment specialists.
- Customized enrichment strategies according to specific experimental objectives.
Related services
To further achieve a comprehensive and in-depth phosphorylation analysis, Creative Proteomics can combine either IMAC or MOAC with orthogonal fractionation techniques, such as reversed-phase liquid chromatography (RPLC), hydrophilic interaction chromatography (HILIC), electrostatic repulsion-hydrophilic interaction chromatography (ERLIC), and strong cation exchange (SCX). In addition to phosphopeptide enrichment service, we also offer acetylated peptide enrichment service, ubiquitinated peptide enrichment service, glycopeptide enrichment service, and histone isolation and enrichment service. If you are interested in our services, do not hesitate to contact us. We will provide you with a strong technical support to save your heart and effort.
References
- Li, Jiaran, et al. "Comprehensive Evaluation of Different TiO2-Based Phosphopeptide Enrichment and Fractionation Methods for Phosphoproteomics." Cells 11.13 (2022): 2047.
- Chang, Y-Y., H. Li, and H. Sun. "Immobilized metal affinity chromatography (IMAC) for metalloproteomics and phosphoproteomics." Inorganic and Organometallic Transition Metal Complexes with Biological Molecules and Living Cells. Academic Press, 2017. 329-353.
Our products and services are for research use only.


 Fig. 1 Overview of phosphopeptide enrichment using immobilized metal affinity chromatography (IMAC). (Chang, Y-Y., H. Li, and H. Sun, 2017)
Fig. 1 Overview of phosphopeptide enrichment using immobilized metal affinity chromatography (IMAC). (Chang, Y-Y., H. Li, and H. Sun, 2017)