Diphthamide, a unique post-translationally modified amino acid, has emerged as a focal point of interest in the realm of molecular biology. This extraordinary modification, located at a highly conserved site on eukaryotic elongation factor 2 (eEF2), plays a pivotal role in cellular processes, making it a subject of intense scrutiny and investigation.
Structure and Biosynthesis
Diphthamide is characterized by its complex structure, comprising a diphthine moiety linked to the histidine residue of eEF2 through an amide bond. The biosynthesis of diphthamide is a multi-step process involving multiple enzymes, with the key players including Dph1-Dph7. The intricate interplay of these enzymes orchestrates the transformation of unmodified histidine into the modified diphthamide residue.
Role of Diphthamide
- Protein Synthesis Regulation. Diphthamide's primary function revolves around the modulation of protein synthesis. By residing on eEF2, this modification plays a crucial role in the translocation step of translation. Diphthamide modification ensures the fidelity and efficiency of protein synthesis, underscoring its indispensability for cellular homeostasis.
- Anti-Tumor Properties. Recent research has shed light on the anti-tumor properties associated with diphthamide. Studies indicate that diphthamide deficiency may lead to increased susceptibility to certain cancers. Understanding the intricacies of diphthamide's involvement in tumorigenesis holds promise for the development of targeted therapeutic interventions.
Analytical Methods of Diphthamide
- Mass Spectrometry. The analysis of diphthamide often involves cutting-edge mass spectrometry techniques. Liquid chromatography-mass spectrometry (LC-MS) has proven instrumental in detecting and quantifying diphthamide in complex biological samples. This high-sensitivity approach allows for precise identification, enabling researchers to delve into the dynamics of diphthamide modification.
- Nuclear Magnetic Resonance (NMR). NMR spectroscopy stands as another invaluable tool for diphthamide analysis. Providing insights into the three-dimensional structure and dynamics of diphthamide-modified proteins, NMR aids in unraveling the functional consequences of this modification. The combination of mass spectrometry and NMR offers a comprehensive analytical approach, enhancing our understanding of diphthamide's role in cellular processes.
Applications of Diphthamide Analysis
- Drug Development. The pivotal role of diphthamide in protein synthesis regulation positions it as a potential target for drug development. Investigating compounds that modulate diphthamide levels or its associated pathways holds promise for designing novel therapeutics, especially in the context of diseases where dysregulation of protein synthesis is implicated.
- Diagnostic Biomarker. Diphthamide's association with certain cancers underscores its potential as a diagnostic biomarker. Monitoring diphthamide levels in clinical samples may provide valuable insights into disease progression and aid in personalized treatment strategies.
Our Service Advantages
Diphthamide does not operate in isolation; its functions are intricately linked with other post-translational modifications. Understanding the crosstalk between diphthamide and neighboring modifications is imperative for a holistic comprehension of cellular processes.
Diphthamide stands as a molecular linchpin in cellular processes, regulating protein synthesis and exhibiting potential implications in disease contexts. Creative Proteomics is dedicated to protein research, we utilize advanced analytical methods to help researches understande its applications at the forefront of molecular biology. As we continue to decipher the complexities of diphthamide, the potential for groundbreaking discoveries in both basic science and clinical applications remains ripe for exploration.
Translational fidelity and growth of Arabidopsis require stress-sensitive diphthamide biosynthesis
Journal: Nature Communications
Published: 2022
Background
Diphthamide is a post-translationally modified histidine residue found in the polypeptide chain of the eukaryotic protein synthesis elongation factor (eEF2). Diphthamide is the target of the Corynebacterium diphtheriae diphtheriae toxin, which catalyzes the ribosylation of ADP, leading to the irreversible inactivation of eEF2, the termination of protein synthesis and cell death. In yeast, mouse, and humans, Diphthamide biosynthesis is regulated by seven genes, from DPH1 to DPH7.
Results
The authors identified DPH1 (At5g62030) in Arabidopsis that is homologous to the amino acid sequences of ScDph1 and HsDPH. And they verified that the DPH1 protein is required to maintain normal plant growth by deletion mutants dph1-1 and dph1-2(Figure 1).
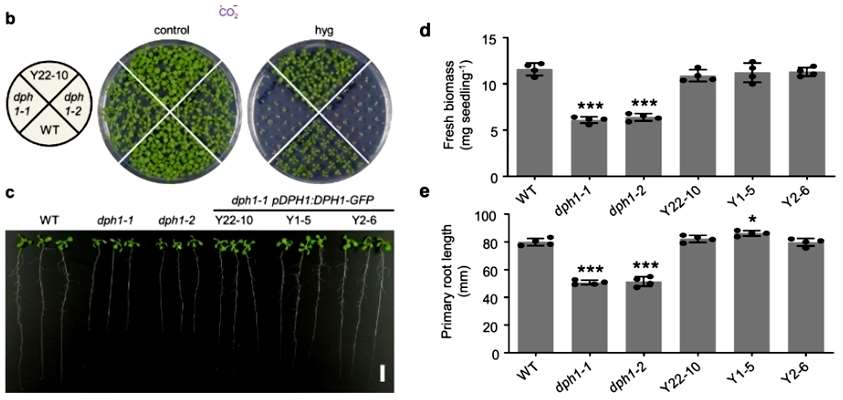 Figure 1
Figure 1
The subcellular localization of AtDPH1 in the cytoplasm was observed with confocal microscopy, and the conserved histidine residues modified by diphthamide corresponded to H700 of Arabidopsis eEF2. unmodified EEF2 could be detected by immunoblotting with a specific antibody in DPH1-1 and DPH1-2 mutants, but not in the wild type. the authors again detected EEF2 by mass spectrometry to detect post-translational modifications of EEF2. Diphthamide modification of EEF2 was detected in the wild type, whereas only unmodified spectral fragments were detected in the dph1-1 and dph1-2 mutants. Detection of ribosomal shift mutations in Arabidopsis revealed 47% and 39% higher mutation rates in the dph1-1 and dph1-2 mutants, respectively, compared with the wild type. The results suggest that the diphthamide modification of eEF2 is conserved and that AtDPH1 is required for the diphthamide modification of eEF2 and helps to ensure the precision of Arabidopsis translation(Figure 2).
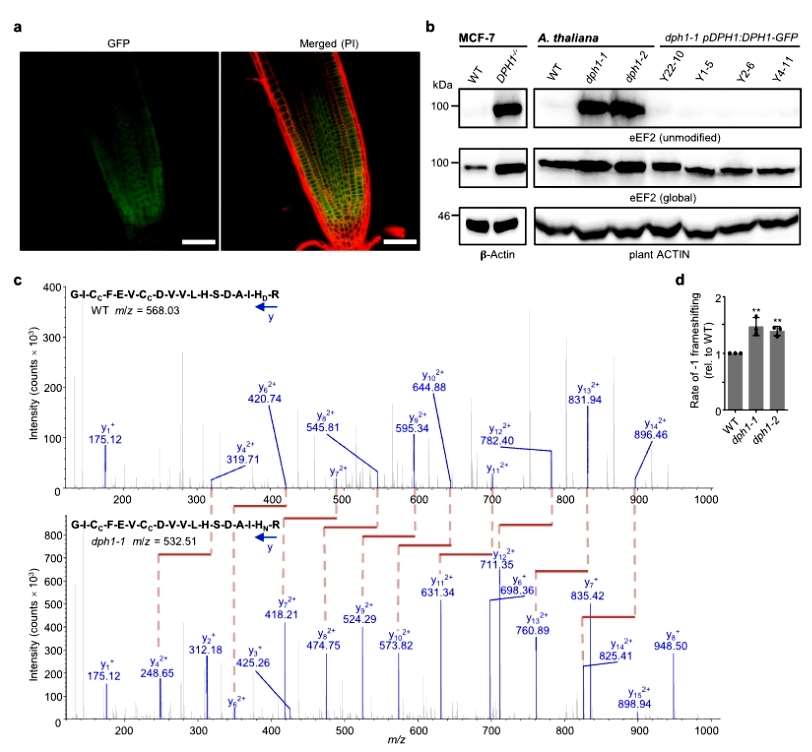 Figure 2
Figure 2
The authors further found experimentally that Arabidopsis dph1 mutant cells have reduced proliferation and decreased TOR kinase activity, and autophagy is activated. Transcriptome sequencing of wild-type and mutant seedlings revealed that 267 genes were up-regulated and 242 genes were down-regulated in the mutant compared with the wild type. The up-regulated genes were significantly enriched in biological processes related to abiotic stresses, such as responses to cold and desiccation, and the down-regulated genes were enriched in pathways related to biotic stresses, such as defense responses to pathogens and resistance-related biological processes(Figure 3).
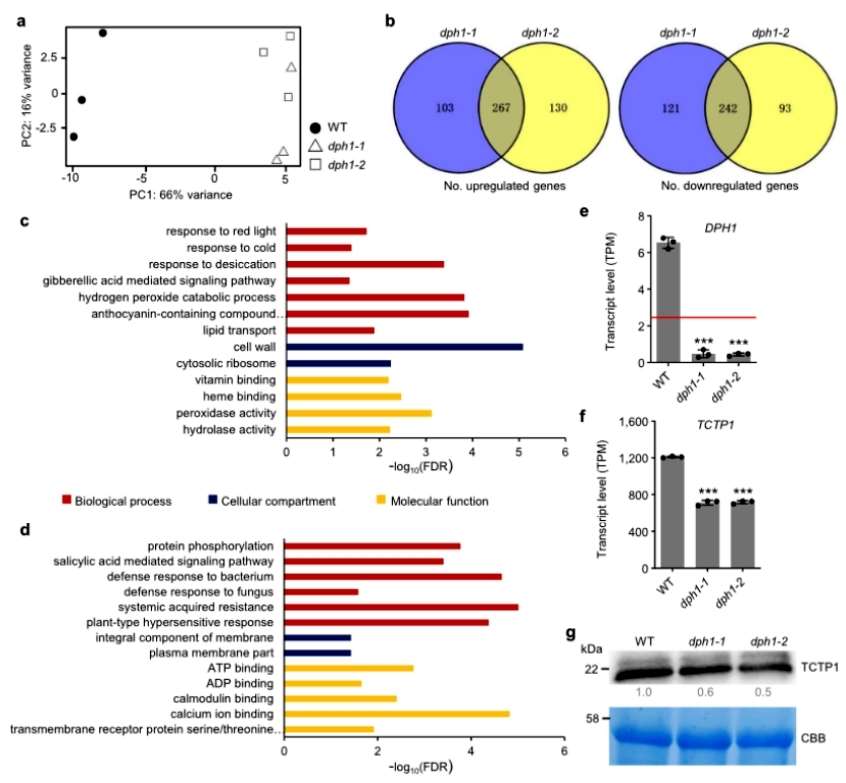 Figure 3
Figure 3
Conclusion
The research demonstrates the conservation of diphthamide modification in Arabidopsis thaliana and its dependence on the AtDPH1 protein. Arabidopsis dph1 mutants exhibit increased ribosomal -1 frameshifting-error rates, resulting in shorter roots and smaller rosettes due to a reduction in formed cells compared to the wild type. Additionally, the study reveals that TOR kinase activity is attenuated, and autophagy is activated in dph1 mutants. Under abiotic stress, diphthamide-unmodified eEF2 accumulates in wild-type seedlings, particularly in response to heavy metal excess, suggesting a conserved mechanism with human cells. Overall, the findings suggest that diphthamide plays a crucial role in the functionality of the translational machinery in plants, influencing growth regulation through TOR kinase activity and autophagy.
Reference
- Zhang H, Quintana J, Ütkür K, et al,. Translational fidelity and growth of Arabidopsis require stress-sensitive diphthamide biosynthesis. Nat Commun. 2022 Jul 11;13(1):4009.


 Figure 1
Figure 1 Figure 2
Figure 2 Figure 3
Figure 3