Overview of Protein Phosphorylation Significance
Phosphorylation is a process wherein the phosphate groups of ATP molecules are transferred to specific sites on proteins via the enzyme, protein kinase. The majority of cellular processes are, in fact, regulated by reversible protein phosphorylation, with at least 30% of proteins undergoing phosphorylation modifications. The binding sites for phosphorylation occur at Serine (Ser), Threonine (Thr), and Tyrosine (Tyr) residues located on the protein. As a consequence of phosphorylation regulation, both the morphology and functionality of the cell undergo transformation.
Phosphorylation, a reversible process, plays a central role in nearly all physiological and pathological processes, encompassing cellular signal transduction, tumorigenesis, metabolism, neuronal activity, muscle contraction, and cellular proliferation, development, and differentiation, among others. The seminal research contributions on reversible protein phosphorylation as a biological regulatory mechanism earned Edwin G. Krebs and Edmond H. Fischer the Nobel Prize in Physiology or Medicine in 1992.
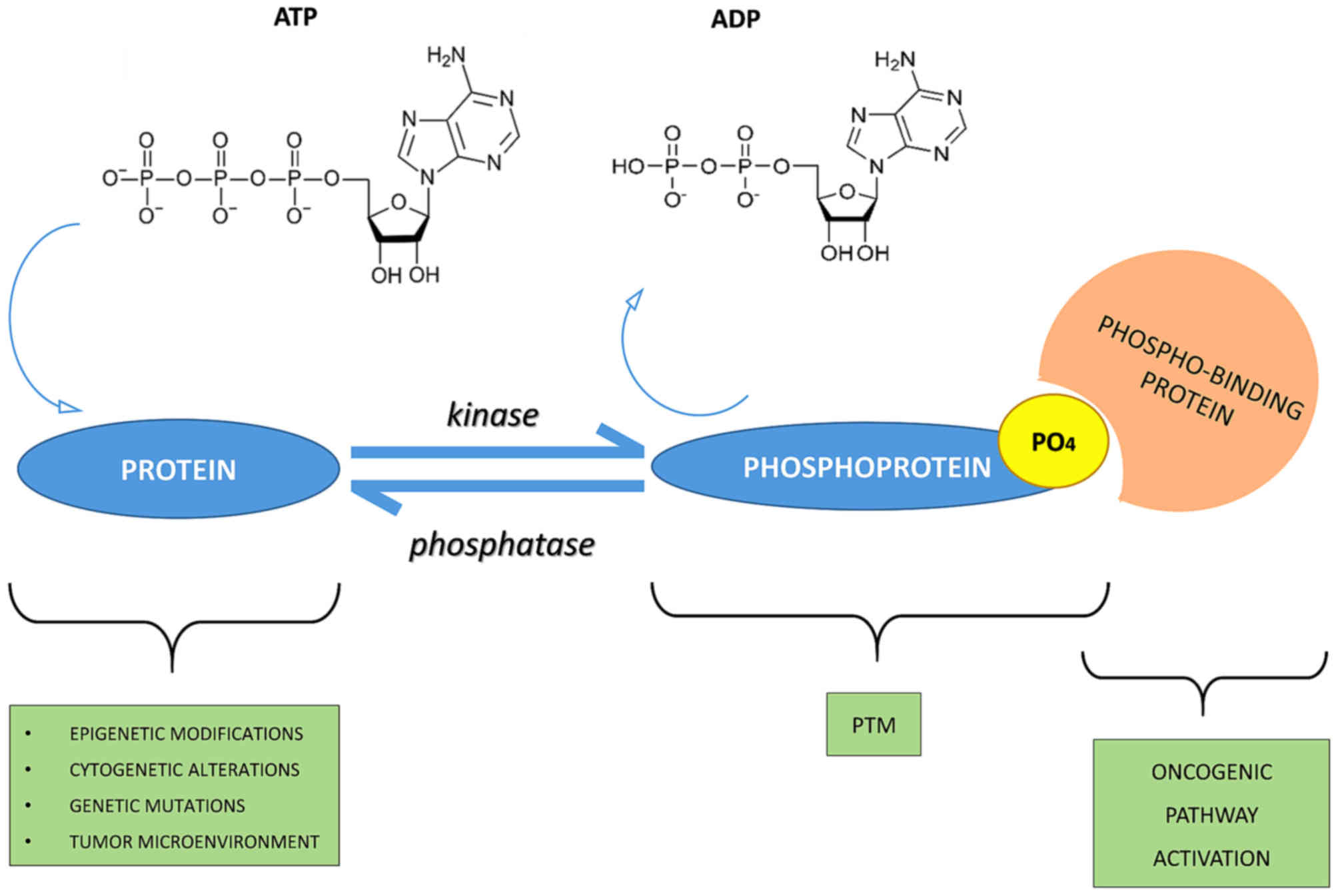 Phospho-signaling networks (Fatima Ardito et al,. 2017)
Phospho-signaling networks (Fatima Ardito et al,. 2017)
Roles of Protein Phosphorylation in Disease Pathways: From Cancer to Viral Replication
In the process of cellular signal transduction, some hormones or cytokines, serving as cellular signals, dualistically bind to cell membrane receptors or intracellular receptors and are activated by kinases. Concurrently, the hormones or signaling factors are phosphorylated along with the kinase activation, inducing a signaling effect within the cells.
In cancer research, it has been discovered that the phosphorylation of microtubule proteins might lead to cancer. Cells utilize a "last checkpoint strategy" (LCP) to control cell apoptosis, by assembling α-tubulin proteins containing nitrotyrosine onto the microtubule, which can lead to microtubule dysfunction and eventual cell apoptosis. However, if the tubulin tyrosine ligase (TTL) gets phosphorylated, cells might be able to "evade" the LCP-controlled cell apoptosis, eventually developing into cancer cells.
In the study of DNA metabolism, it was found that DNA damage in cells can lead to hyperphosphorylation of the N-terminus of the 32 kD subunit of human replication protein A (RPA). This helps regulate DNA metabolism and promote DNA repair. Data shows that over-phosphorylation can lead to conformational changes in RPA, reducing the activity of DNA replication, but not affecting DNA repair.
Varied Impact of Protein Phosphorylation on Cellular Functions and Structures
Cabrejos et al. investigated the phosphorylation effect catalysed by the vertebrate protein kinase CK2 on general transcription factors TFIIA, TFIIE, TFIIF and RNA Polymerase II (RNAP II). The results indicated that the major subunits of TFIIA, TFIIE, and TFIIF were phosphorylated by CK2, and 214 kD and 20.5 kD subunits of RNAP II were phosphorylated. The phosphorylation of TFIIA, TFIIF and RNAP II promoted the formation of a complex on the TATA box of the Ad-MLP promoter. Among them, the phosphorylation of TFIIF facilitated transcription, while the phosphorylation of RNAP II showed significant transcriptional inhibition.
Maile and team in their study on histone phosphorylation found that Drosophila Universal Transcription Factor TAF1's C-terminal kinase domain (CTK) phosphorylates evolutionarily conserved Serine 33 (H2B-S33) on histone H2B. The phosphorylation of H2B-S33 in the promoter of cell cycle regulator gene string and segmentation gene giant is correlated with transcriptional activity.
Evans et al. discovered in their research on Hepatitis C virus (HCV) non-structural protein 5A (NS5A) that the effective replication of RNA requires an interaction between HCV NS5A and human vesicle-associated membrane protein-associated protein A (hVAP-A). Further investigations revealed that adaptive mutations inhibiting the phosphorylation of NS5A can facilitate its binding with hVAP-A. Moreover, the phosphorylation of NS5A has a negative regulatory effect on the replication of the filtrable virus RNA.
In their study, Jones et al. unveiled a novel mechanism regulating SHP-1: the C-terminal Ser591 of SHP-1 undergoes phosphorylation, catalyzed by protein kinase C alpha (PKCα). This phosphorylation process negatively regulates SHP-1 activity, evidenced by a diminished ability of phosphorylated SHP-1 to dephosphorylate Vav1 tyrosine residues in vitro, consequentially leading to an enhancement in substrate tyrosine phosphorylation.
In their research, Li Yanmei et al. discovered that protein phosphorylation modifies the local conformation of the protein through hydrogen bonding interactions. They focused on the impacts of phosphorylation modifications in the C-terminal structural domain of the c-Fos protein and found that phosphorylation at S362 triggers alterations in the local conformation, subsequently impacting the stability of the turn structure. Further, they utilized Matrix Assisted Laser Desorption Ionization - Time of Flight Mass Spectrometry (MALDI-TOF MS) to analyse post-source decay (PSD) of peptides derived from phosphorylated proteins. This provided insights into patterns of PSD in MALDI-TOF MS for differently phosphorylated amino acids, thereby revealing useful information about phosphorylation sites in the peptides.
References
- Ficarro S B, McCleland M L, Stukenberg P T, et al. Phosphoproteome analysis by mass spectrometry and its application to Saccharomyces cerevisiae. Nature Biotechnology, 2002, 20(3): 301~305
- Krupa A, Preethi G, Srinivasan N. Structural modes of stabilization of permissive phosphorylation sites in protein kinases: distinct strategies in Ser/Thr and Tyr kinases. J Mol Biol, 2004, 339: 1025~1039
- Idriss H T. Three steps to cancer: how phosphorylation of tubulin, tubulin tyrosine ligase and P-glycoprotein may generate and sustain cancer. Cancer Chemother Pharmacol, 2004, 54: 101~10
- Binz S K, Sheehan A M, Wold M S. Replication protein A phosphorylation and the cellular response to DNA damage. DNA Repair, 2004, 3: 1015~1024[DO : 2~10
- Cabrejos M E, Allende C C, Maldonado E. Effects of phosphorylation by protein kinase CK2 on the human basal components of the RNA polymerase Ⅱ transcription machinery. Journal of Cellular Biochemistry, 2004, 93
- Maile T, Kwoczynski S, Katzenberger R J, et al. TAF1 activates transcription by phosphorylation of Serine 33 in histone H2B. Science, 2004, 304: 1010~1014
- Evans M J, Rice C M, Goff S P. Phosphorylation of hepatitis C virus nonstructural protein 5A modulates its protein interactions and viral RNA replication. Proc Natl Acad Sci USA, 2004, 13038~13043
- Jones M L, Craik J D, Gibbins J M, et al. Regulation of SHP-1 tyrosine phosphatase in human platelets by serine phosphorylation at its C terminus. J Biol Chem, 2004, 279(39): 40475~40483:
- Huang Z P, Li Y M, Luo S Z, et al. Identification of phosphorylation site of phosphopeptide by MALDI-PSD mass spectrometry. Molecular & Cellular Proteomics, 2004, 3(10): 131
Our products and services are for research use only.

 Phospho-signaling networks (Fatima Ardito et al,. 2017)
Phospho-signaling networks (Fatima Ardito et al,. 2017)