The Role of Mitochondria in Aging: Unraveling Mechanisms of Cellular Senescence
Online InquiryMitochondria, the powerhouse of the cell, have emerged as pivotal players in the intricate symphony of aging processes. Aging, a complex and inevitable biological process, is accompanied by a myriad of molecular changes that impact cellular function and tissue integrity. Mitochondria, with their vital role in energy production and cellular homeostasis, have garnered considerable attention as both contributors and responders to the aging process.
The Intricate Role of Mitochondria in Cellular Aging and Senescence
Mitochondria, the energy-producing powerhouses of cells, have emerged as central players in the fascinating realm of cellular aging and senescence.
Mitochondrial Energetics
At the heart of cellular function lies the intricate dance of energy production orchestrated by mitochondria. These organelles are responsible for generating adenosine triphosphate (ATP), the universal energy currency of cells. Some studies have shown that as cells age, mitochondrial efficiency declines, resulting in diminished ATP synthesis. This energetic shortfall contributes to a wide array of age-related cellular impairments, ranging from reduced metabolism to impaired tissue repair.
Oxidative Stress and the Mitochondrial Connection
Mitochondria's essential role in energy production comes with a cost—oxidative stress. While generating ATP, mitochondria produce reactive oxygen species (ROS) as natural byproducts. Accumulated ROS inflict damage on cellular components, particularly mitochondrial DNA (mtDNA), as well as proteins and lipids.
Mitochondrial DNA and the Fragile Thread of Cellular Youth
Mitochondrial DNA (mtDNA) holds a unique position in the cellular landscape. Unlike nuclear DNA, mtDNA lacks protective histones and is exposed to the relentless assault of ROS generated in close proximity. The accumulation of mutations and deletions in mtDNA impairs the synthesis of essential mitochondrial proteins, leading to diminished energy production and a cascade of cellular dysfunctions.
Inflammation and the Mitochondrial Nexus
Inflammation, a hallmark of aging, is intimately intertwined with mitochondrial function. Dysfunctional mitochondria release pro-inflammatory signals, contributing to chronic inflammation observed in aging tissues. Conversely, chronic inflammation can disrupt mitochondrial dynamics and impair their function, creating a vicious cycle that accelerates cellular senescence.
Mitochondrial Dynamics and Senescence
Mitochondria are not static entities, they undergo dynamic fusion and fission processes, crucial for maintaining a healthy mitochondrial population. Some researches has revealed that imbalances in mitochondrial dynamics contribute to cellular aging. With age, the delicate equilibrium between fusion and fission is disrupted, leading to the accumulation of fragmented and dysfunctional mitochondria. These aberrant mitochondria are less efficient at producing ATP and release elevated levels of ROS, further fueling the aging process.
Mitophagy
Mitophagy, the process of selectively degrading damaged mitochondria, plays a pivotal role in cellular rejuvenation. A number of related studies have shown that studies have demonstrated that impaired mitophagy is a key driver of aging. With advancing age, the efficiency of mitophagy declines, leading to the accumulation of dysfunctional mitochondria. These impaired organelles not only contribute to energy deficits but also release pro-inflammatory signals, perpetuating the cycle of cellular senescence.
Unveiling Mechanisms of Declining Mitochondrial Function in Aging
The decline in mitochondrial function with advancing age is a hallmark of the aging process, intricately linked to the accumulation of cellular damage.
Mitochondrial DNA Damage
Mitochondrial DNA (mtDNA) is exceptionally susceptible to damage due to its proximity to the reactive oxygen species (ROS) generating machinery within the mitochondria. Some studies have elucidated how ROS-induced lesions, such as single-strand breaks and base modifications, accumulate in mtDNA over time. As these lesions accumulate, they compromise the fidelity of mtDNA replication, leading to the creation of mutant copies.
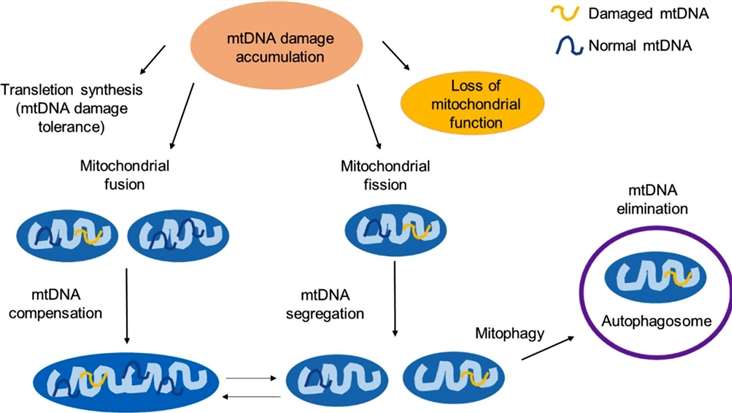 The mitochondrial response to unrepaired DNA damages (Rong Z, et al,. 2021)
The mitochondrial response to unrepaired DNA damages (Rong Z, et al,. 2021)
Vicious Cycle of mtDNA Damage
The damage to mtDNA sets off a chain reaction that perpetuates mitochondrial dysfunction. Some researches has uncovered a vicious cycle wherein mutant mtDNA leads to the synthesis of defective mitochondrial proteins, further impairing the function of the electron transport chain (ETC). This impairment results in a heightened production of ROS as electrons "leak" from the ETC, exacerbating mtDNA damage. This self-perpetuating cycle amplifies mitochondrial dysfunction and contributes to cellular aging.
The Guardians of mtDNA Integrity
Mitochondria possess an array of DNA repair pathways aimed at maintaining mtDNA integrity. Some researches meticulous investigations have illuminated the central role of these repair mechanisms in mitigating the impact of mtDNA damage. Base excision repair (BER) and nucleotide excision repair (NER) are among the repair pathways that correct specific types of lesions. However, as cells age, these repair mechanisms become less efficient, leading to the accumulation of unrepaired mtDNA lesions.
The Enigma of Mitochondrial Replication Errors
Unlike nuclear DNA, mtDNA lacks the protective armor of histones and is exposed to the corrosive environment of the mitochondria. Some researches has unveiled that during mtDNA replication, errors are more likely to occur, resulting in the insertion of incorrect nucleotides. Over time, these replication errors lead to the expansion of mutant mtDNA populations, contributing to the heterogeneity of mitochondrial genomes within a single cell—known as heteroplasmy.
mtDNA Heteroplasmy and Cellular Fate
The extent of mtDNA heteroplasmy—a mix of mutant and wild-type mtDNA—plays a pivotal role in determining cellular fate. A threshold level of mutant mtDNA must be reached before cellular dysfunction becomes evident. Once this threshold is surpassed, the delicate balance of mitochondrial function is disrupted, leading to impaired energy production, elevated ROS production, and the initiation of cellular senescence.
Crosstalk Between Mitochondrial Dysfunction and Senescence
Mitochondrial dysfunction contributes to the release of pro-inflammatory signals, known as senescence-associated secretory phenotype (SASP), promoting the establishment of senescent cells. Conversely, senescent cells display further mitochondrial impairment, creating a feedback loop that exacerbates the aging process.
Case Study of Mitochondria in Aging
Background
Aging is an inevitable biological process characterized by the gradual decline in physiological function and an increased susceptibility to diseases. Understanding the mechanisms underlying aging has been a longstanding quest in the field of biology. Over the years, accumulating evidence has underscored the pivotal role of mitochondria in the aging process. Mitochondria, the energy-producing organelles within cells, have garnered attention not only for their role in generating adenosine triphosphate (ATP) but also for their involvement in reactive oxygen species (ROS) production, mitochondrial DNA (mtDNA) maintenance, and the regulation of cell death pathways. Mitochondrial dysfunction has been recognized as a hallmark of aging. As cells age, mitochondrial function tends to deteriorate, leading to various consequences such as increased oxidative stress, impaired ATP production, and a decline in cellular quality control mechanisms.
Sample
Numerous studies have contributed to our understanding of mitochondria's role in aging. One illustrative example comes from a study (Trifunovic, et al. 2005), which utilized a mouse model with a specific mtDNA mutation. This mutation led to a proofreading deficiency in mtDNA replication, resulting in the accumulation of deleterious mutations. As a consequence, the mice displayed accelerated aging phenotypes, including hair graying, weight loss, and a shortened lifespan. This experimental model highlights the significance of mtDNA integrity in the aging process and underscores the role of mitochondria in maintaining cellular health.
Technical Approach
To investigate the intricate relationship between mitochondria and aging, researchers employ a range of technical approaches, each shedding light on different aspects of this complex phenomenon.
- Mitochondrial DNA Analysis: One fundamental technique involves the sequencing and analysis of mtDNA. Researchers examine mtDNA mutations and deletions that accumulate over time in aged tissues. High-throughput sequencing technologies have allowed for the identification of specific mtDNA mutations associated with aging and age-related diseases, providing insights into the role of mitochondria in these processes.
- Mitochondrial Function Assessment: Functional assays measure mitochondrial activities such as ATP production, mitochondrial membrane potential, and oxygen consumption rates. These assays provide quantitative data on how mitochondrial function changes with age and in response to various interventions.
- Reactive Oxygen Species (ROS) Measurement: ROS, byproducts of mitochondrial respiration, can damage cellular components. Researchers employ techniques like flow cytometry and fluorescent probes to quantify ROS levels and assess their impact on cellular aging.
- Animal Models: Animal models, including mice and nematodes, are crucial for investigating the role of mitochondria in aging. Genetic manipulations, like the aforementioned mtDNA mutations, allow researchers to simulate mitochondrial dysfunction and study its effects on the aging process.
Results
Research findings consistently support the central role of mitochondria in aging:
- Mitochondrial DNA Mutations: Studies have identified specific mtDNA mutations that accumulate with age and contribute to mitochondrial dysfunction. These mutations can lead to impaired electron transport chain function and increased ROS production, ultimately accelerating the aging process.
- Oxidative Stress: Mitochondria are a major source of ROS production. Elevated ROS levels can damage cellular components, including proteins, lipids, and DNA, leading to cellular dysfunction and contributing to aging-related pathologies.
- Mitochondrial Quality Control: Age-related declines in mitochondrial quality control mechanisms, such as autophagy and mitophagy, result in the accumulation of damaged mitochondria. This further exacerbates cellular dysfunction and the aging phenotype.
- Therapeutic Implications: The emerging understanding of mitochondria's role in aging has opened avenues for potential interventions. Caloric restriction, exercise, and pharmaceutical agents targeting mitochondrial function are being explored as strategies to mitigate the aging process and age-related diseases(Ghosh-Choudhary SK, et al.2021).
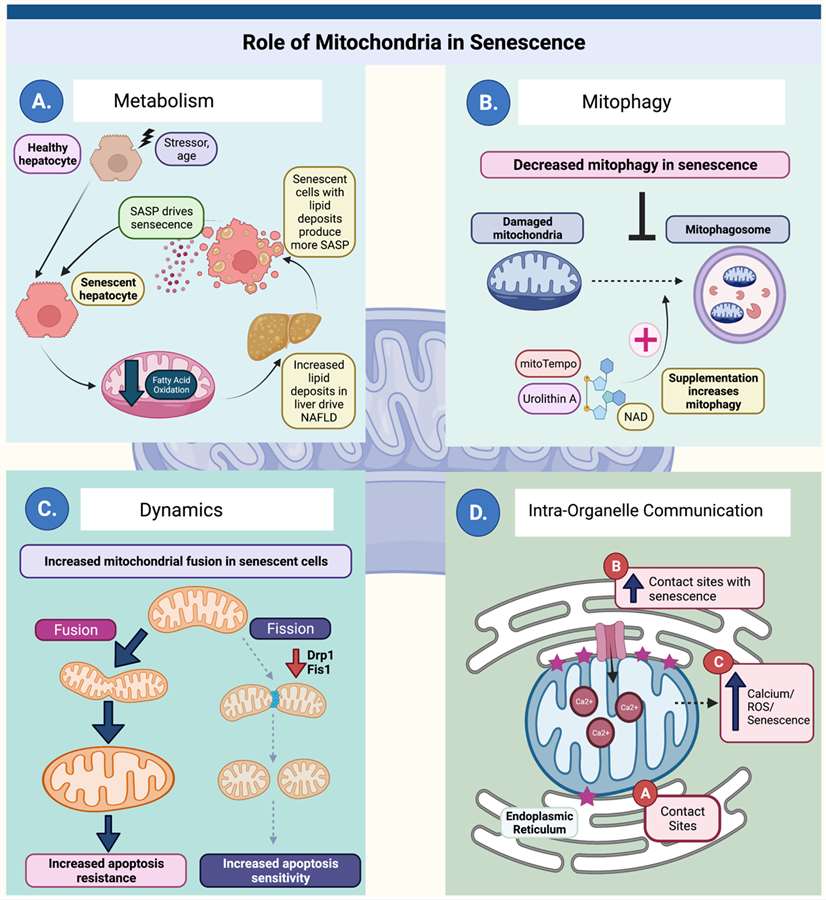 Mitochondria participate in multiple aspects of senescence biology (Ghosh-Choudhary SK, et al, 2021)
Mitochondria participate in multiple aspects of senescence biology (Ghosh-Choudhary SK, et al, 2021)
Creative Proteomics' groundbreaking research reveals the complex network connecting mitochondria, cellular senescence and aging. Insights gained from years of meticulous experimentation have elucidated the mechanisms of declining mitochondrial function and its profound impact on the aging process. We offer comprehensive mitochondrial research services including mitochondrial proteomics, mitochondrial lipidomics, mitochondrial functional analysis, mitochondrial damage detection, and mitochondrial autophagy, among others.
References
- Rong Z,Tu P,Xu P, et al. The Mitochondrial Response to DNA Damage. Front Cell Dev Biol. 2021;9:669379.
- Trifunovic A, Hansson A, Wredenberg A, et al. Somatic mtDNA mutations cause aging phenotypes without affecting reactive oxygen species production. Proc Natl Acad Sci U S A. 2005 ;102(50):17993-8.
- Ghosh-Choudhary SK, Liu J, Finkel T. The role of mitochondria in cellular senescence. FASEB J. 2021 ;35(12):e21991.
Related Services
* For Research Use Only. Not for use in diagnostic procedures.