Exosomes as Drug Carriers
Online InquirySince their discovery, exosomes have been potentially used as drug carriers for various diseases and cancers due to their unique properties such as stability, tumor targeting, and biocompatibility. As natural carriers, exosomes have not only been utilized to deliver RNA and proteins but also for the delivery of therapeutic small molecules.
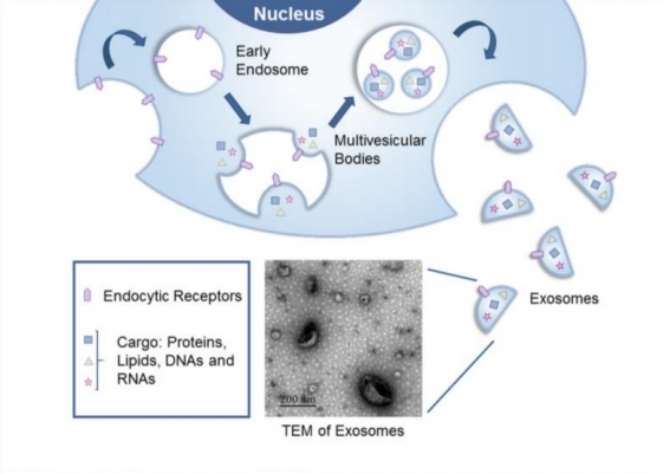 Fig. 1 Exosomes arise from the endocytic pathway that begins with the invagination of the plasma membrane to form an endosome. (Jiang, Xin-Chi, and Jian-Qing Gao, 2017)
Fig. 1 Exosomes arise from the endocytic pathway that begins with the invagination of the plasma membrane to form an endosome. (Jiang, Xin-Chi, and Jian-Qing Gao, 2017)
Challenges in drug delivery by exosomes
Because of their good biocompatibility, low immunogenicity, and toxicity as well as their ability to cross biological barriers such as the blood-brain barrier (BBB), they are proposed as powerful gene and drug delivery systems. Despite these benefits, large-scale production and purification of exosomes, as well as standard and efficient methods for gene and drug loading, are extremely difficult and significant issues.
- Large-scale exosome production
Different culture conditions and methods can affect the properties and biology of cell-derived exosomes. There is a lack of approved methods for large-scale cell culture and the production of exosomes with constant properties and characteristics.
- Exosome isolation and purification
Although many methods for isolating and purifying exosomes have been developed, exosome isolation and purification are major problems in using exosomes as delivery systems. To date, there is still a need to develop isolation and purification technologies based on purity and scalability.
- Macromolecular drug loading
Due to their length and charge, mRNA is very difficult to be loaded in the exosomes. In order to achieve more satisfactory and practical results for loading large molecules in exosomes, the development of some loading methods and corresponding studies are still needed.
Our Service Support
Creative Proteomics is committed to providing innovative solutions to power your biological research. We proudly offer a range of exosome research and analytical services, involving exosome production, isolation and purification, drug loading, and so on.
| Exosome analysis services | |
|---|---|
| Exosome isolation and purification | Sucrose gradient centrifugation |
| Polymer-based exosome enrichment method | |
| Immunomagnetic bead method | |
| Size exclusion chromatography method | |
| Exosome identification | Nanoparticle tracking analysis (NTA) |
| Electron microscopy analysis | |
| Western blot | |
| Exosome marker assay | Isolation and enrichment of exosomal CD9, CD63, CD81, TSG101, HSP70 proteins |
| Exosome surface protein identification and quantitative analysis | |
| Exosome engineering | Exosome labeling and tracking |
| Cargo loading | |
| Engineered exosome production | |
| Cargo loading assessment | |
| Exosomes labeling and tracking | Exosome fluorescent labeling |
| Fluorescence exosome purification | |
| Fluorescent exosome concentration labeling | |
| Exosome multiomics analysis | Exosome proteomics analysis: Exosome protein profile identification Exosome protein composition analysis Exosome protein expression level analysis Exosome protein differential expression analysis |
| Exosome metabolomics analysis: Exosome differential metabolite screening Qualitative and quantitative analysis of target metabolites/metabolic pathways | |
| Exosome lipidomics analysis: Exosome lipid composition and level analysis Differential expression analysis of exosomal lipid molecules Qualitative and quantitative analysis of targeted lipid molecules | |
| Exosomal biogenesis and identification | Our capabilities will provide strong support for the study of exosome biogenesis and its identification. |
| Exosomal cargo and loading mechanism | Our services can greatly help the study of exosomal cargo and loading mechanism, facilitating the diagnostic and therapeutic applications of exosomes. |
| Exosome function research | In vitro analysis of the function of exosomes In vivo analysis of the function of exosomes |
Our capability can greatly promote the application of exosomes as carriers for various genes and drug delivery, including
As single-stranded DNA molecules, ASOs typically consist of 12-25 nucleotides that regulate RNA function via several mechanisms. In addition to being a powerful molecular tool for protein and RNA biology research, ASOs represent a highly selective therapeutic strategy for many diseases associated with gene expression disorders.
In recent years, siRNA has received increasing attention as the preferred mechanism for inducing gene silencing due to its high specificity, efficacy, ease of synthesis, and low side effects. siRNAs are double-stranded RNA molecules ranging in length from 19 to 25 nucleotides. RNA interference (RNAi) via siRNAs, which is similar to many miRNAs that trigger sequence-specific catalytic mRNA degradation.
As a common non-coding RNA, miRNA is known to bind to partially complementary mRNA sequences to promote their degradation or translation inhibition, thereby regulating various physiological and pathological activities. In particular, there is growing evidence that miRNAs are highly associated with cancer development and/or progression.
In vitro transcribed (IVT) mRNAs have recently shown great potential as a new drug class to deliver genetic information. Synthetically modified mRNAs provide the basics for a broad range of potential applications, including infectious disease vaccines for current and future pandemics, cancer immunotherapies, and the manufacturing of monoclonal antibodies
circRNA is one of the emerging nucleic acid drugs. The special loop structure of circRNA not only prevents being degraded and improves circRNA expression and expression time, but also allows repeated administration. However, there are some challenges with the exosome-based delivery of exogenous circRNAs. On one hand, circRNAs have low circular efficiency. On the other hand, macromolecular circRNAs are difficult to load into the exosomes. To address these issues, the researchers have built relevant lentiviral vectors to properly and efficiently cyclize circRNAs and transfect them into target cells and load them into exosomes.
Intracellular protein delivery is an enormous obstacle to its development into a universal therapy. Advances in intracellular protein delivery will facilitate the development of protein drugs. Exploiting exosomes for delivering macromolecular proteins has shown potential. Various studies have applied exosomes to the delivery of peptides and proteins, such as enzymes, cytoskeletal proteins and transmembrane proteins.
The physicochemical properties of the drug molecules and the fate of the drugs in the in vivo environment play an important role in achieving the desired bioavailability. As natural carriers, exosomes have not only been utilized to deliver RNA and proteins but also for the delivery of therapeutic small molecules, including chemotherapeutic drugs and plant-derived bioactives.
Major benefits of our services
- Cutting-edge technology and advanced platform
- High-throughput facility and strict analysis workflow
- Powerful exosome research and analysis competence
- Customized services from experienced experts
References
- Zhang, Yi, et al. "Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications." International journal of nanomedicine 15 (2020): 6917.
- Jiang, Xin-Chi, and Jian-Qing Gao. "Exosomes as novel bio-carriers for gene and drug delivery." International journal of pharmaceutics 521.1-2 (2017): 167-175.
* For Research Use Only. Not for use in diagnostic procedures.