Extracellular Vesicle Analysis: Size, Concentration, and Zeta Potential
Online InquiryThe particle size, concentration, and Zeta potential of extracellular vesicles (EVs) are crucial parameters reflecting their biophysical characteristics and biological functions. The size of EVs depends on the originating cells, carriers, and biogenesis pathways, ranging in diameter from 30nm to 150nm. The concentration of EVs varies from a few to thousands per milliliter, depending on their source and isolation methods. Zeta potential measures the surface charge, influencing EV stability, aggregation, and interactions with other particles or cells. Generally, the Zeta potential of EVs falls within the range of -10 to -70mV, depending on the composition of the culture medium and pH.
The particle size, concentration, and Zeta potential of EVs are closely related to their applications in diagnosis, treatment, and biotechnology. For instance, the particle size of EVs affects their distribution, clearance rate, and targeting within the human body. EV concentration indicates sample quantity and quality, as well as the efficiency and purity of isolation methods. The Zeta potential of EVs influences their stability in different formulations and interactions with drugs or large biomolecules. Therefore, accurately and precisely measuring these parameters is essential for determining the characteristics of EVs and optimizing their applications across various fields.
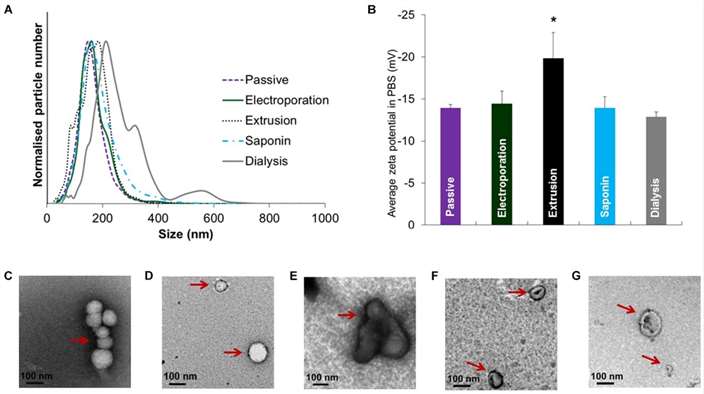 Size distribution, zeta potential and morphology of EVs after drug loading with various methods (Chen et al., 2021).
Size distribution, zeta potential and morphology of EVs after drug loading with various methods (Chen et al., 2021).
Particle Size of Extracellular Vesicles
There are various methods available for measuring the particle size of EVs, including nanoparticle tracking analysis (NTA), electron microscopy, single-particle interferometric reflectance imaging sensor technology, atomic force microscopy, and resistive pulse sensing. These methods can provide different information about EV size distribution, morphology, and concentration. However, each method has its own strengths and limitations, such as accuracy, precision, sensitivity, resolution, sample preparation, and cost. Therefore, the choice of method depends on the purpose as well as the availability of equipment and expertise.
One of the most commonly used methods for measuring the size of EVs is NTA. NTA uses a laser beam to illuminate particles in a fluid, and a CCD camera records the Brownian motion of the particles. The particle size is calculated based on their diffusion coefficients, which are inversely proportional to their diameter. Currently available NTA devices can measure the size and concentration of EVs in the range of 30 to 1000 nm, with a resolution of 10 nm. In addition, NTA can provide information about the polydispersity and aggregation of EVs. However, NTA also has limitations, as it requires relatively high concentrations of EVs (at least 10^8 particles per milliliter) and may be influenced by other particles or contaminants present in the sample.
Another widely used method for measuring the size of EVs is electron microscopy (EM), which uses electron beams to generate particle images on thin films or grids. Electron microscopy can provide high-resolution images of EV morphology, structure, and size distribution. EM can measure the size of EVs in the range of 30 to 150 nm, with a resolution of 1 nm. However, EM detection requires a considerable amount of time for sample preparation, such as fixation, dehydration, staining, or coating, which may alter the characteristics or integrity of the EVs themselves. Additionally, EM methods require expensive and sophisticated equipment, as well as operators with relevant expertise, making them less suitable for large-scale or routine analyses.
Other methods available for measuring EV size include single-particle interferometric reflectance imaging sensor technology, dynamic light scattering, atomic force microscopy, and resistive pulse sensing. Single-particle interferometric reflectance imaging sensor technology uses interference of light waves to determine the size and refractive index of EVs. Dynamic light scattering detects changes in scattered light due to the Brownian motion of particles in solvent molecules to discriminate particle sizes. Atomic force microscopy scans the surface of EVs with a sharp tip to measure their height and diameter. Resistive pulse sensing uses electric current to detect changes in resistance as EVs pass through a small pore. These methods offer high sensitivity and accuracy for EV size measurement but may have drawbacks such as low throughput, complexity, or limited sample volume.
There are various methods available for measuring the particle size of EVs, each with its own advantages and disadvantages. The choice of detection method primarily depends on the research question, sample quality, and available resources.
Exosomes Particle Concentration
There are various methods available for measuring the particle concentration of EVs, including NTA, flow cytometry (FCM), resistive pulse sensing, EM, dynamic light scattering (DLS), and microfluidic devices. These methods can provide different information about the quantity and density of EVs in a sample. Each method has its own strengths and limitations, such as accuracy, precision, sensitivity, resolution, sample preparation, and cost. Therefore, the choice of method depends on the purpose as well as the availability of equipment and expertise.
One of the most commonly used methods for EV concentration measurement is NTA. Another widely used method is flow cytometry (FCM), which uses laser beams to detect light scatter or fluorescence emission from individual particles passing through a narrow channel. The particle concentration is calculated based on the number of events recorded by the detector. FCM can measure EV concentrations ranging from 10^6 to 10^9 particles per milliliter, with a sensitivity of 103 particles per milliliter. With specific antibodies or probes, FCM can also provide information about surface markers and phenotypes of EVs. However, FCM requires complex sample preparation, such as labeling, dilution, or filtration, which may alter the characteristics or integrity of EVs. Other methods available for measuring EV concentration include tunable resistive pulse sensing (TRPS), EM, dynamic light scattering (DLS), and microfluidic devices.
Zeta Potential of Extracellular Vesicles
Zeta potential is a measure of the surface charge of EVs and can influence their stability, aggregation state, and interactions with other particles or cells. Several methods can be used to measure the zeta potential of EVs, such as electrophoresis and tunable resistive pulse sensing (TRPS). These methods provide different information about the electric field and mobility of EVs in solution.
Electrophoresis is one of the most commonly used methods for measuring the zeta potential of EVs. It induces the movement of charged particles in a solution under the influence of an electric field. The zeta potential of particles is calculated based on their electrophoretic mobility, which is directly proportional to their surface charge and inversely proportional to their size and viscosity. Electrophoresis can measure the zeta potential of EVs, typically ranging from -10 to -70mV, depending on factors such as the composition of the medium and pH.
Another widely used method for measuring the zeta potential of EVs is tunable resistive pulse sensing (TRPS). TRPS uses electric current to detect changes in resistance as EVs pass through a small pore. The zeta potential of EVs is calculated based on their transit time and frequency, which are influenced by their size and surface charge. TRPS can measure the zeta potential of EVs in the range of -10 to -50mV, depending on pore size and voltage. It's important to note that TRPS requires careful calibration and optimization of parameters such as pore size, voltage, pressure, and temperature.
Reference
- Chen, Huizhi, et al. "Exosomes, a new star for targeted delivery." Frontiers in Cell and Developmental Biology 9 (2021): 751079.
Related Services
* For Research Use Only. Not for use in diagnostic procedures.