Deep Blood 4D-DIA Proteomics
Blood is the most commonly tested specimen in the clinic, and its protein changes serve as markers to indicate the onset and progression of a variety of diseases. From glutathione in routine physical examinations to protein indicators such as AFP and CA125 in cancer diagnosis, they provide essential and important information for clinical diagnosis. In recent years, with the advancement of mass spectrometry technology, the screening of new blood markers through omics methods has become an increasingly important research application. Mass spectrometry-based blood high-throughput proteomics is gradually becoming a powerful tool for studying blood proteins, and has become an indispensable research tool in tumor research, immune diseases, cardiometabolic diseases, and reproductive fields.
Because of its special protein structure, blood makes it difficult to detect at the level of omics. There are two main reasons: first, compared with general tissue and cell samples, the dynamic range of blood protein distribution is wide (spanning 10 orders of magnitude), which means that some proteins are very high and others are extremely low. This is a huge challenge for instrument detection, and it is difficult for any instrument to span such a large detection range. Second, these proteins are not only highly variable in content, but also very unevenly distributed. In the plasma/serum samples, serum albumin (HSA) alone accounted for about 50% of all proteins; the collection of the first 22 proteins, moreover, accounted for 99% of all proteins; the remaining tens of thousands of proteins together accounted for less than 1%.
Deep Blood 4D-DIA Proteomics Analysis
Deep blood proteomics is an omics technology based on the advantages of nanoparticle-specific enrichment of low-abundance proteins in blood samples, combined with 4D-DIA (data-independent acquisition method) to achieve in-depth analysis of blood samples. Among them, nano-mass spectrometry proteomics is currently an innovative research method for blood proteomics. Its special enrichment method greatly increases the identification depth of low-abundance proteins in blood samples, which can be used for biomarker screening of blood samples and realize tumor liquid biopsy.
Through the optimization of the sample preparation method, the mass spectrometry method and the upgrade of the data analysis process, we can achieve the identification of more than 2,000 proteins in a single blood sample, the highest identification number has reached more than 3,000, and the identification depth of multi-sample detection can reach more than 4,000, greatly improving the detection depth of blood proteins, and the coverage of low-abundance proteins reached more than 90%, which is very suitable for clinical research for the deep identification of blood needs.
Technical Advantages
Medium and low-abundance protein coverage: specific adsorption of low- and medium-abundance proteins
High-depth identification: the number of identifications is as high as 1500-2000+
First-class mass spectrometry platform and software: 4D-DIA mass spectrometry platform (timsTOFpro2); Spectronaut new version software
Sample Requirements
| Sample Type | Blood | Plasma/Serum |
| Quantify | 1 mL | 0.2-0.5 mL |
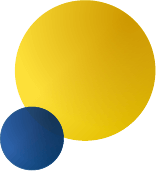
Data Analysis
References:
- Rapid, deep and precise profiling of the plasma proteome with multi-nanoparticle protein corona. Nat Commun.
- Nano–Bio Interactions in Cancer: From Therapeutics Delivery to Early Detection. Acc Chem Res.
* For Research Use Only. Not for use in the treatment or diagnosis of disease.



 4D Proteomics with Data-Independent Acquisition (DIA)
4D Proteomics with Data-Independent Acquisition (DIA)