4D-Phosphoproteomics Services
Protein phosphorylation modification is one of the most important covalent modifications in living organisms. It is the most distributed and studied modification which occurs by transferring the phosphate group of ATP to the amino acid (Ser, Tyr, Thr) side chain of proteins catalyzed by kinase. The reversible process of phosphorylation and dephosphorylation regulates all life activities, including cell proliferation, development, differentiation, signal transduction, apoptosis, neural activity, muscle contraction and tumorigenesis. Statistically, the molecular mass of phosphorylated amino acids covalently attached to the phosphate group increased by 79.97 Da. Thus, mass spectrometry can be used to accurately determine whether a mass shift of 79.97 Da has occurred in the molecular weight to detect phosphorylated peptides and modification sites. D-label-free phosphorylated proteomics is based on a new generation of timsTOF Pro mass spectrometer for differential phosphorylation proteomics analysis.
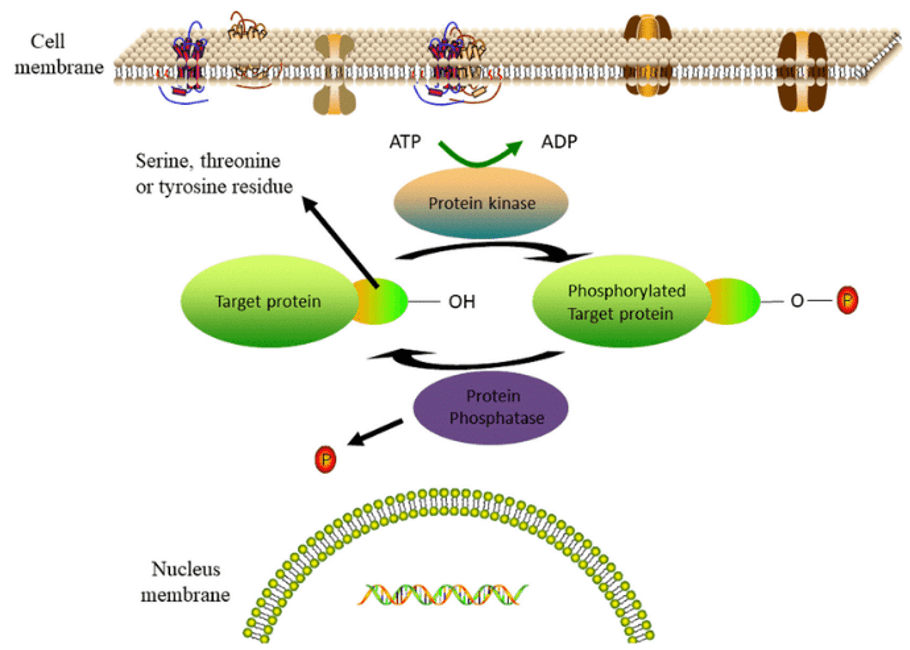 The catalytic cycle for protein phosphorylation by a protein kinase (Te Li 2019)
The catalytic cycle for protein phosphorylation by a protein kinase (Te Li 2019)
Our 4D-Phosphoproteomics Services
Creative Proteomics provides 4D-label free phosphoproteomics, using the ion flow separation to effectively distinguish modified tautomeric peptides according to the molecule's shape and cross-sectional area properties. This technology significantly improves the depth of modifications identification and achieve a comprehensive improvement of proteomics in terms of coverage depth, sensitivity and throughput with less loading volume and faster scanning speed.
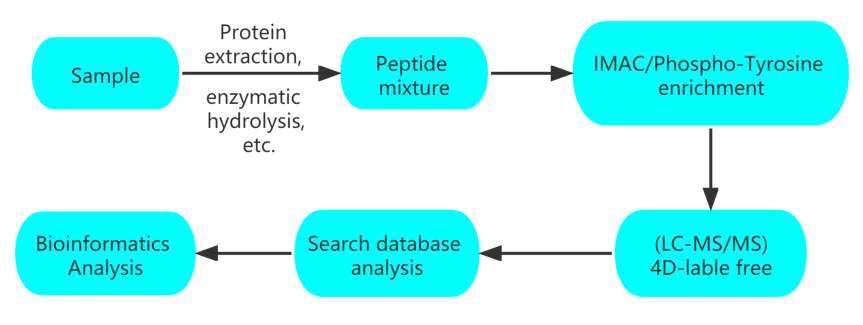
Workflow
Applications
- Plant development and resistance to stress.
- Infection and immunity research.
- Aging regeneration and degenerative diseases.
Data Analysis
| Standard Data Analysis |
| Analysis of total protein identification quantity |
Protein identification and quantitative result statistics, Venn diagram analysis, number of different results statistics, volcano map, cluster heat map |
| Protein function analysis |
Subcellular localization, domain analysis, motif analysis, GO function analysis, KEGG pathway analysis, interaction network analysis |
| Quality control analysis |
Peptide relative molecular mass distribution, peptide sequence length distribution |
| Advanced Data Analysis |
| Protein Gene Chromosome Localization |
Obtain genes encoding proteins distribution on chromosomes |
| WGCNA Analysis |
Predict functional clustering and network interactions by protein expression level; correlate with phenotype data to obtain key proteins or protein complexes that influence phenotype |
| Trend Cluster Analysis |
Obtain protein expression trend patterns |
| Molecular Typing |
Molecular typing for large cohort samples |
| Survival Curve Analysis |
Study the relationship between influencing factors, survival time and outcome |
| ROC Curves |
Evaluate predictive accuracy by combining specificity and sensitivity, such as biomarker impact assessment on tumor grade |
Technical Advantages
- The support of 4D technology improves quantification accuracy and identification depth, more suitable for trace samples;
- Overcoming the limitation of labeled quantitative proteomics techniques in terms of sample size, which is flexible and convenient;
- Cutting-edge technology-based on a new generation of mass spectrometry platform (Bruker timsTOF pro);
- High speed - nearly doubled detection speed;
- Extensive experience - over 10 years of protein platform service experience, with leading high-level project articles in quantity and quality;
- Data assurance - stable and reliable data results;
- "One-stop" service - PTMs analysis, verification, upstream and downstream joint analysis.
Sample Requirements
We can quickly extract proteins from various samples and design personalized experimental schemes according to different experimental purposes based on our unique protein extraction technology. Specific requirements are as follows:
| Sample Type |
Protein |
# of Cells |
Animal Tissue |
Plant Tissue |
Blood |
Urine |
Serum |
Microbes |
| Quantify |
100 ug |
1×107 cells |
1 g |
200 mg |
1 mL |
2 mL |
0.2-0.5 mL |
Dry weighed: 200 mg |
Recommended Combination
(1) Proteome + Phosphorylation: comprehensive and precise dissection of regulatory networks.
(2) Phosphorylation + metabolomics: open up the phenotype-mechanism research pathway.
Reference:
- Synergistically Bifunctional Paramagnetic Separation Enables Efficient Isolation of Urine Extracellular Vesicles and Downstream Phosphoproteomic Analysis. ACS Appl Mater Interfaces. 2021.
* For Research Use Only. Not for use in the treatment or diagnosis of disease.

 The catalytic cycle for protein phosphorylation by a protein kinase (Te Li 2019)
The catalytic cycle for protein phosphorylation by a protein kinase (Te Li 2019)

 4D Proteomics with Data-Independent Acquisition (DIA)
4D Proteomics with Data-Independent Acquisition (DIA)