Matrix-assisted laser desorption/ionization (MALDI) is a technique for soft ionization of mass spectrometry that uses a laser energy absorbing matrix to create ions from large molecules with minimal fragmentation. It has been widely used to measure the molecular weight of biomacromolecules such as peptides, proteins, nucleic acids, molecular weight distribution of polymers, and oligomer analysis. MALDI mass spectrometry is characterized by high sensitivity, wide application range, and simple operation. It has extended the traditional mass spectrometry technology mainly for small molecule research to analyze the range of highly polar, hardly volatile, and thermally unstable samples.
The MALDI method is divided into three steps. First, the sample is mixed with the appropriate substrate material and applied to the metal plate. Secondly, the pulse laser irradiates the samples, triggering the ablation and desorption of samples and matrix materials. Finally, the analyte molecules are ionized by protonation or deprotonation in the hot plume of the ablation gas, and then accelerated to the mass analyzer to analyze them.
The Principle of MALDI
For heat-sensitive compounds, if they are heated very quickly, they can be prevented from being thermally decomposed. The MALDI technique is similar to this principle: in a tiny region, in a very short time interval (on the order of ns), the laser provides high-intensity pulsed energy to the analyte on the target, allowing it to desorb and ionize in an instant without producing thermal decomposition. MALDI is a mass spectrometry ionization method for directly vaporizing and ionizing non-volatile samples, but its ionization mechanism is still unclear. There are two possibilities that ions are formed in the solid state, and the laser irradiation is only a simple release or produced by a laser-induced ion-molecule reaction.
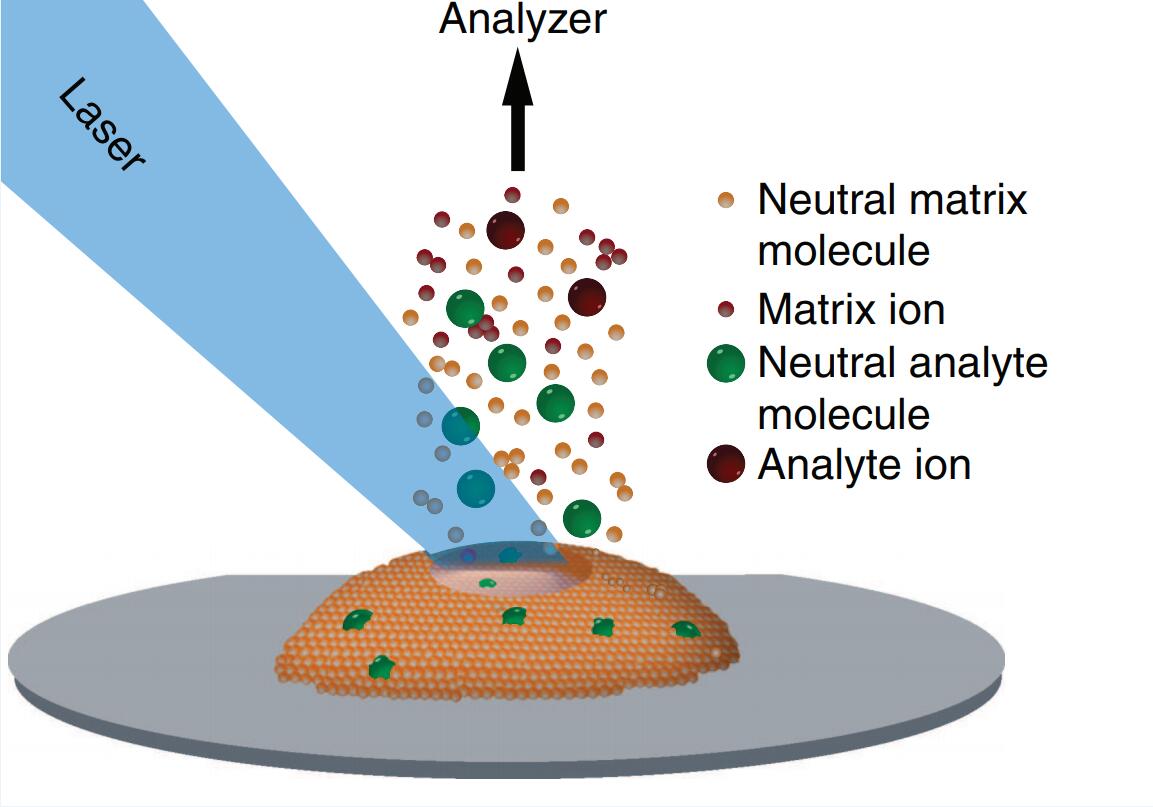 Figure 1. A schematic diagram of MALDI (RC, Dreisewerd, K. 2007.)
Figure 1. A schematic diagram of MALDI (RC, Dreisewerd, K. 2007.)
Matrix of MALDI
The matrix is a substance that coexists with the sample to be tested and absorbs the incident laser light to prevent direct irradiation thereof to destroy the sample. The sample to be tested is dispersed in a matrix at a high dilution ratio (matrix: sample = 10000:1), and the matrix effectively absorbs the energy of the pulsed laser of a certain wavelength, and then uniformly transmits it to the sample, so that it is instantaneously vaporized and ionized. In addition, a large amount of matrix effectively disperses the sample to be tested, thereby reducing the interaction between the molecules of the sample to be tested.
The choice of matrix is one of the most important steps in MALDI analysis. The ideal matrix generally has the following properties: strong electron absorption at the laser wavelength used; better vacuum stability, lower vapor pressure, and good miscibility with the analyte in the solid state. In MALDI technology, the matrix has a special effect on the analysis of the sample: dilute the sample to dissociate the clustered macromolecule; protect the sample, the matrix absorbs the laser energy and then transfers it to the sample to avoid direct laser irradiation of the sample and lead to decomposition of the sample molecules. The choice of matrix depends primarily on the wavelength of the laser used, followed by the nature of the object being analyzed. Commonly used substrates are niacin, 2,5-dihydroxybenzoic acid and mesonic acid.
Most MALDIs use solid (crystalline) organic matrices, but these matrices produce corresponding background peaks in low mass regions, which interfere with the characterization of small molecule compounds. Recently, a series of new matrix materials have been reported which can reduce or remove matrix interference, such as high molecular weight porphyrins, surfactant inhibiting substrates, inorganic materials, carbon nanotubes and porous silicon. In addition, the addition of matrix additives to the matrix solution can improve the analytical results and greatly improve the sensitivity, reproducibility, resolution and signal suppression of desorption.
Characteristics of MALDI
Compared with other mass spectrometry ionization techniques such as electron bombardment ionization and chemical ionization, MALDI technology has the following characteristics:
- It can ionize some samples that are difficult to ionize by other ionization techniques (especially biomacromolecules), and obtain complete ionization products without obvious fragments;
- It has a dominant single-charged molecular ion peak, and simple mass spectrum, which is suitable for analysis of multi-component samples;
- The requirements for sample processing are not strict, and even untreated can be directly analyzed;
- MALDI can be coupled to Fourier transform ion cyclotron resonance mass spectrometry (FTICR-MS), and its resolution is further improved.
Applications of MALDI
MALDI is a soft ionization technique that can create ions from large molecules with minimal fragmentation. It has been applied to the analysis of biomolecules, large organic molecules, etc.
- Peptides and proteins. MALDI technology is widely used in the determination of molecular weight and purity of peptides and proteins, such as bovine carbonic anhydrase, melittin, bovine insulin, bovine insulin B chain, gramicidin S, myoglobin, cytochrome C, trypsinogen, etc. For complex biological mixtures, especially for samples containing a certain concentration of salt and buffer, fast atom bombardment (FAB) and electrospray ionization (ESI) analysis will produce inhibition of peptide ion signals. But the use of MALDI-MS to analyze protein hydrolysates does not present these problems. The combination of MALDI with enzymatic or chemical degradation is a good way to study protein structure and confirm the structural consistency of genetically engineered drugs with natural components. In addition, MALDI technology can be used to determine the location of disulfide bonds in proteins.
- Nucleotide. The nucleotides are polar and thermally unstable, and direct laser desorption is prone to fragmentation. MALDI shows great potential in nucleotides analysis, especially in oligonucleotide analysis. Early analysis was limited to oligonucleotides of less than 50 bases due to the inability of the normal matrix to completely desorb ionized DNA. Becker studied the application of a cryogenic frozen solution matrix in DNA and demonstrated that the MALDI-MS method can be used for the analysis of more than 100 bases.
- Carbohydrate. MALDI has been used in the analysis of carbohydrate, glycolipids, and phospholipids. Papac et al. proposed to use a 2,5-dihydroxybenzoic acid (DHB) as a matrix to measure neutral sugar components in positive ion mode using reflective MALDI. For the matrix, the acidic sugar component was determined in a negative ion mode using linear MALDI.
- Polymer compound. The determination of the molar mass and distribution of polymer compounds plays an extremely important role in the research and development of polymer materials. MALDI-MS determines the molar mass of polymer materials with the following characteristics: the measurement is independent of the sample or Mark-Houwink constant; the absolute molar mass is measured instead of the relative molar mass, and the accuracy is higher than the methods of light scattering and membrane permeation; the molar mass distribution is obtained, not just an average value; structural information of the terminal groups can be provided at the same time; it can be used for the molar mass determination of polymers such as copolymers and graft polymers.
MALDI mass spectrometry is characterized by high sensitivity, wide application range, and simple operation. It has extended the traditional mass spectrometry technology mainly used for small molecule research to analyze the range of highly polar, hardly volatile, and thermally unstable samples.
We have briefly introduced the MALDI, a type of ionization methods, which can help you understand more about mass spectrometry. At Creative Proteomics, we have the professional mass spectrometry platform, which contains advanced instruments. By using mass spectrometry, Creative Proteomics can provide different services to meet various requirenments, including:
References
- Karas; et al. Ion Formation in MALDI: The Cluster Ionization Mechanism. Chemical Reviews. 2003, 103 (2): 427–440.
- Chantw, D; et al. Accuratemass measurements for peptide and protein mixtures by using matrix-assisted laser desorption / ionization Fourier transform mass spectrometry. AnalChem. 2002, 74: 5282-5289.
- Keki, S; et al. Matrix-assisted laserdesorption /ionizationmass spectrometric study of the oligomers formed from lactic acid and dipheny lmethane diisocyanate. Macromolecules. 2001, 34(21): 7288-7293.
- Rhoads, D.D; et al. The presence of a single MALDI-TOF mass spectral peak predicts methicillin resistance in staphylococci. Diagn Microbiol Infect Dis. 2016, 86(3): 257-261.
- Vogne, C; et al. A simple, robust and rapid approach to detect carbapenemases in Gram-negative isolates by MALDI-TOF mass spectrometry: validation with triple quadripole tandem mass spectrometry, microarray and PCR. Clin Microbiol Infect. 2014, 20:O1106.
- Sakarikou, C; et al. Rapid detection of carbapenemase-producing Klebsiella pneumoniae strains derived from blood cultures by Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry (MALDI-TOF MS). BMC Microbiol. 2017, 17(1): 54.
- Lau, A; et al. A Rapid Matrix-Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry-Based Method for Single-Plasmid Tracking in an Outbreak of Carbapenem-Resistant Enterobacteriaceae. Journal of Clinical Microbiology. 2014, 52(8): 2804–2812.
- Dreisewerd, K. Recent methodological advances in MALDI mass spectrometry. Anal Bioanal Chem. 2014 , 406(9-10): 2261-78.
- Cramer, R; Dreisewerd, K. UV Matrix‐Assisted Laser Desorption/Ionization: Principles, Instrumentation, and Applications. Encyclopedia of Mass Spectrometry. 2007, 6: 646‐661.






