Glycosylation increases the structural complexity and functional diversity of protein molecules, it is not only affects the folding, structure, transportation, localization and maintenance of biological activity of the modified protein, in further as well regulates various physiological processes, such as cell growth, division, differentiation, identification, and adhesion through specific recognition between sugar and protein. Among them, N-linked glycoprotein is ubiquitous on the cell surface and various body fluids. Cell surface glycoprotein is supposed to be a pivotal drug target agent, for instances, humoral glycoprotein is the main source of disease-related biomarkers. The quantitative study of N-glycan proteomics under various physiological and pathological conditions enable to elucidate the function of protein glycosylation and is of great significance new biomarkers identification in cancer prevention and diagnosis.
According to the substitution of mannose residues, the N-linked glycans can be roughly divided into three groups: complex, high-mannose, and hybrid type of structures. The complex types contain fucose, sialic acid, and α-linked galactose as terminating sugars. The high-mannose type contains multiple mannose residues and the hybrid-type mannose on one branch of the core structure, and a lactosamine sequence on the other. At present, the commonly used N-glycan linkage analysis strategy is to separate and enrich the glycoprotein/glycopeptide, then use enzymatic or chemical methods to dispatch the sugar chain from the glycosylated polypeptide and perform mass spectrometry analysis of the released sugar chain or deglycosylated polypeptide to obtain the overall expression and changes of sugar chains and/or glycoproteins in the sample to be tested.
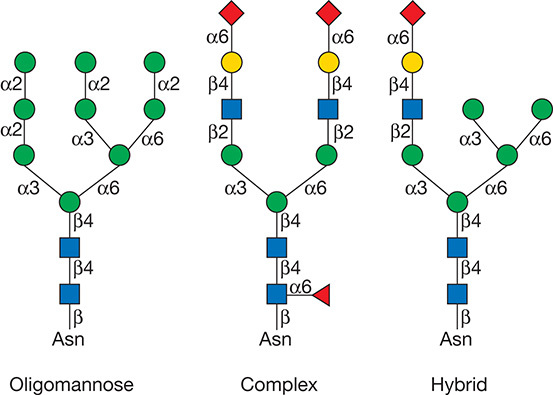 Figure 1. Types of N-glycans (Varki A. et al; 2017).
Figure 1. Types of N-glycans (Varki A. et al; 2017).
Overview of N-Glycan Linkage Analysis
Creative Proteomics provides N-glycan linkage analysis to identify linkage position, monitor the specific glycan species and determine the relative amount of a particular group of glycans. Our N-glycan linkage analysis includes the following steps: i)enzymatic release of the attached glycans; ii)derivatization of the released glycans via reductive amination with aromatic, aliphatic amines or permethylation; iii)analysis of the glycans.
- Sample preparation
- N-glycans release
- Methylation of the released glycans
- Glycans are cleaved with exoglycosidases or acid hydrolysis
- Reduction and derivatization of free hydroxyl groups
- Analyze and quantify by GC-MS
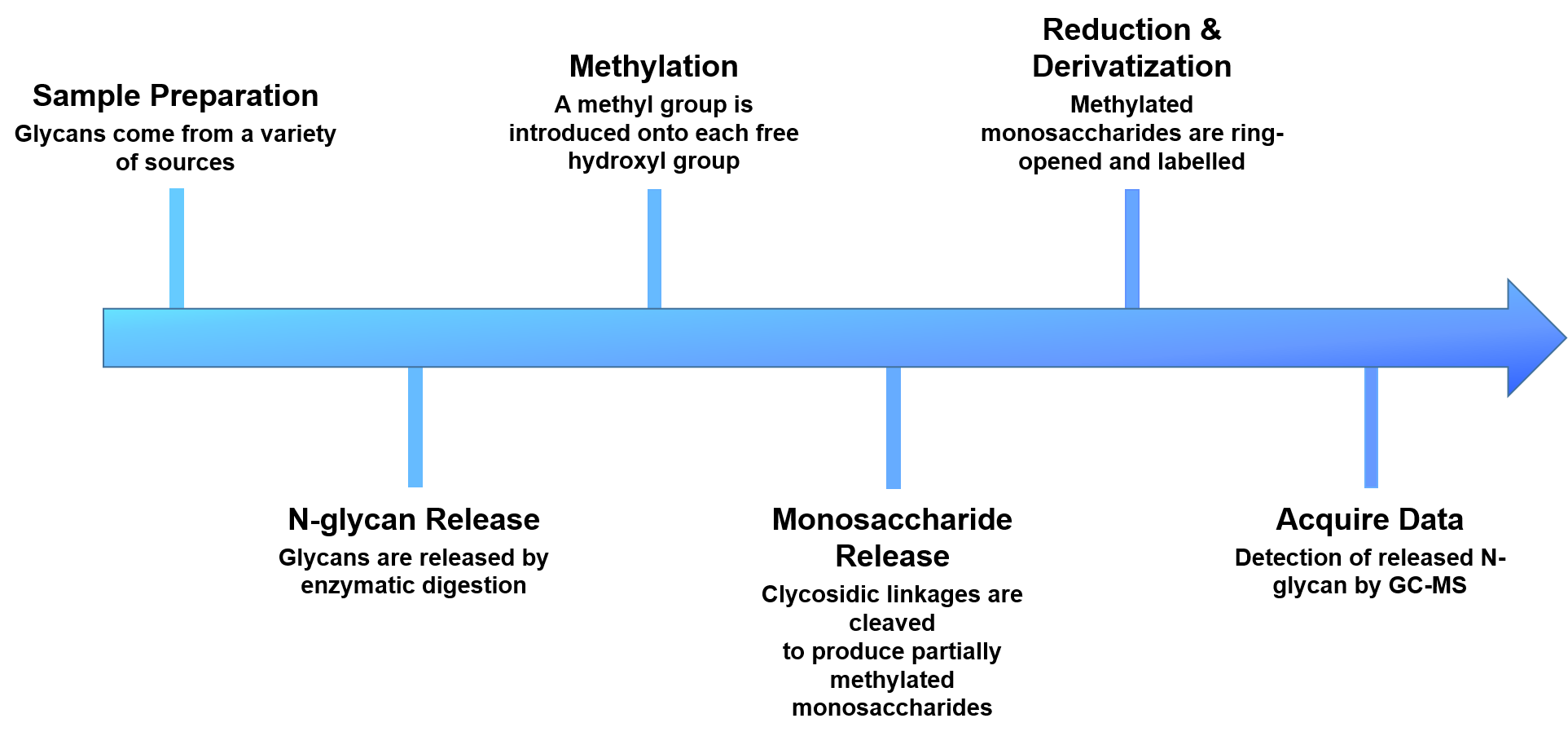 Figure 2. The workflow of our N-glycan linkage analysis.
Figure 2. The workflow of our N-glycan linkage analysis.
The Key Features of Our N-Glycan Linkage Analysis
- Enrichment of N-glycosylated peptides by lectin affinity method with high specificity and good enrichment efficiency
- Highly identified flux, up to 10,000 N-glycosylation sites can be detected at one time
- High detection sensitivity and good repeatability
- Analysis takes a relatively short time
Samples Delivery
- If you are providing a tissue sample, please send the tissue to us with dry ice.
- If you are providing a protein sample, use normal tissue or cell lysate for protein extraction.
Please use a sufficient amount of dry ice to transport, and try to use faster delivery methods to reduce the possibility of sample degradation during transportation. If you have any questions regarding sample delivery, please feel free to contact us and our experts are always available.
With highly experienced bio-scientists and advanced analytical techniques in omics research, Creative Proteomics will support you a range of glycomics and bioinformatics analysis services. Please feel free to contact us and see how we can help you address your problems.
Reference
- Varki A, et al. Essentials of glycobiology [Internet]. 3rd edition. Cold Spring Harbor (NY), 2017.




















