Fast atom bombardment (FAB) is an ionization technique established in 1981, which is widely used as an ion source in mass spectrometry systems. Unlike other ionization techniques, FAB bombards a sample with a neutral atom (Xe, Ar) to ionize the sample. The use of neutral atoms avoids the accumulation of charge in the insulating sample and facilitates ionization of the sample. FAB can successfully determine compounds that are not volatile or derivatized, as well as some polymer compounds. Moreover, FAB is effective for the determination of oligosaccharides, oligopeptides, oligonucleotides, and thermally labile organic compounds and even metal organic compounds.
The Principle of FAB
The FAB directly bombards the surface layer of the sample with a high-speed directional movement of neutral atoms, ionizing the sample to form positive ions [M+H]+, negative ions [M+H]-, and fragment ions. The FAB ion source consists of a cold cathode release ion gun and a collision charge exchange chamber. Ar is ionized in the release ion gun to form Ar+, and then Ar+ forms a high speed Ar+ ion beam under the action of the accelerating voltage and the focusing electrode. The charge exchange chamber is filled with Ar. When the ion beam enters the exchange chamber, the following reaction occurs:
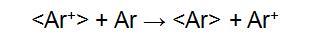
"< >" indicates a particle symbol with rapid directional motion. The fast directional motion of Ar+ acquires electrons from Ar, forming Ar for rapid directional motion. The energy loss of <Ar+> during charge exchange is small, so the resulting <Ar> also has a high energy. <Ar+> is separated in the deflection electric field, and <Ar> is still advancing at a high speed in the original direction, and finally hitting the sample target to ionize the sample:
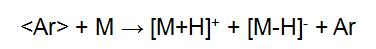
The sample is dissolved in a solvent and applied to a tiny piece of metal in the FAB. The vapor pressure of the solvent used should be low enough to keep the sample in a dissolved state. The solvent used includes glycerin, diethanolamine, ethylene glycol, dimethyl sulfoxide or the like.
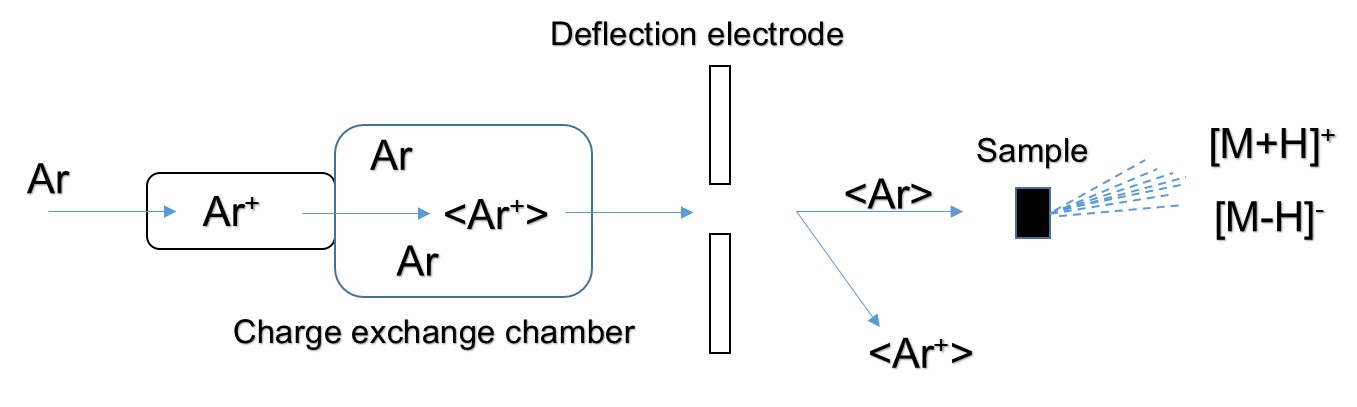 Figure1. Schematic of FAB ion source
Figure1. Schematic of FAB ion source
After the sample is ionized, its positive charge product is mostly present in the form of proton bonding or alkali metal ion binding. Generally only a small amount of alkali metal ions are needed, and a [M+Na]+ or [M+K]+ signal can appear in the mass spectrometer. The [M+Na]+ peak was first observed in samples (polypeptides) with strong basic groups. If the sample is mixed with acid, the signal is strengthened. On the other hand, the anionic product appears in the form of [M-H]-. Acid samples are often ionized into [M-H]-, and salt samples form a charged ion by cleaving a cation or anion. The literature indicates that salt ions are important for determining the molecular weight of a sample and analyzing its structure.
Advantages and Disadvantages of FAB
FAB is very effective for the analysis of non-volatile, thermally unstable polar compounds. The ionization products [M+H]+ and [M-H]- are produced in pairs in FAB, facilitating the analysis of positive ion mass spectrometry and negative ion mass spectrometry. The FAB has high sensitivity and consumes less sample. The minimum measured molecular weight of the sample can be several 10-9g, and the utilization rate of the sample is high. For biologically active substances, the residual sample remains active and can be recycled due to the bombardment of the neutral particles used. In addition, the ionization probability and detection sensitivity of each component in the sample are related to the sample composition. If the energy required for ionization of one component is lower than the other components in the sample, the ionization probability of the component is large.
However, FAB is not suitable for separating samples. It is difficult for FAB to separate the sample with low affinity proton abilities from samples with high affinity proton abilities. Moreover, for samples of mixtures of different volatility, FAB cannot fractionate and ionize the components.
Applications of FAB
As a mass spectrometry ionization source, FAB is suitable for the analysis of highly polar, thermally unstable compounds, especially peptides and proteins. The application of FAB-MS tandem technology provides detailed sample molecular structure information and is widely used in biomedical fields. Among the peptide compounds, FAB successfully analyzes thousands of molecular weight macromolecules, and gives the order and type of amino acids in the polypeptide, and also distinguishes the isomers of the polypeptide, such as cephalin, human gastrin, bovine insulin, bradykinin, adrenocorticotropic hormone and so on. In addition, FAB can measure polar antibiotics such as neomycin, streptomycin, gentamicin, erythromycin, oxytetracycline and so on. In the analysis of compounds for nucleotides and their phosphates, FAB successfully analyzes nicotinamide adenine dinucleotide phosphate (C21H29N7O17P3) and vitamin B12 (C63H88CoN14O14P).
Thermally unstable polar organic compounds are substances that are difficult to vaporize and decompose easily. Based on ionization technology of inert atoms, FAB provides an effective solution for the analysis of such compounds. In conjunction with mass spectrometry, FAB has made significant advances in quantitative analysis and structural analysis. With the continuous development of mass spectrometry technology, the application field of FAB will be further expanded.
We have briefly introduced the FAB, a type of ionization methods, which can help you understand more about mass spectrometry. At Creative Proteomics, we have the professional mass spectrometry platform, which contains different instruments. By using mass spectrometry, Creative Proteomics can provide different services to meet various requirements, including:
References
- Barber M, et al. Fast atom bombardment of solids as an ion source in mass spectrometry. Nature, 1981, 293(5830):270-275.
- Pramanik B N, et al. Structural characterization of the oligosaccharide antibiotics everninomicins by negative ion FAB and ESI/MS. Journal of Antibiotics, 2000, 53(6):640-3.
- Walton T, et al. Differentiation of Isomeric Purine and Pyrimidine Mononucleotides by Fast Atom Bombardment Tandem Mass Spectrometry. Nucleosides & Nucleotides, 2012, 9(7):967-983.
- Barber M, et al. Fast atom bombardment mass spectrometry of bradykinin and related oligopeptides. Biological Mass Spectrometry, 2010, 8(8):337-342.






