Chemical ionization (CI) is a soft ionization technique, which is the direct application of the research results of molecular and ion reactions in analytical chemistry. The earliest ionization technique was electron bombardment ionization (EI). The product obtained by EI has many fragments, which is difficult to analyze. CI, which produced very few fragments, began in the 1950s and has great potential in analytical chemistry.
In the CI process, electrons first bombard the reagent gas to generate reagent ions. The sample molecules are then ionized by reagent ions through molecular and ion reaction pathway. The 1970s was considered a milestone in the development of CI. At that time, researchers solved the shortcomings of CI's work in a vacuum environment, allowing CI to work under atmospheric conditions. Atmospheric chemical ionization provides energy from corona discharge and does not require a vacuum environment, which greatly increases the range of CI applications. Currently, CI has been widely used in the mass spectrometry technique.
The Principle of Chemical Ionization
The principle of CI is to use the reagent ion X+ to react with the analyte molecule A to achieve ionization of the analyte:
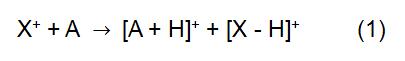
In the above reaction, X+ is derived from the ionized reaction gas. Some common reagent gases include methane, ammonia, water and isobutane. The reaction time and the rate constant (k) in the formula can be obtained by literature or measurement. When the reagent ion X+ is H3O+, the formula (2) is:
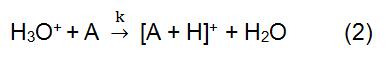
Reagent ion is produced by an ion source. Commonly used ion sources mainly include radiation source, hollow cathode discharge power source, and ordinary glow discharge power source. 210Po and 241Am are the most common sources of radiation. The ionization process begins with the alpha particles emitted by 210Po and 241Am. It has a high energy and can collide with the reagent gas to generate reagent ions and electrons. If the generated electrons are high enough, they may collide with the reagent gas to form new reagent ions and electronics. In the CI using H3O+ as a reagent ion, a hollow cathode discharge power source is commonly used, which can produce 99.5% of H3O+.
According to the conditions of chemical ionization, CI is classified into low pressure chemical ionization (<0.1 Pa), medium pressure chemical ionization (1-2000 Pa), and atmospheric pressure chemical ionization. For different types of chemical reactions, CI is divided into positive chemical ionization and negative chemical ionization. Positive chemical ionization reactions mainly include proton transfer reaction, charge exchange reaction, and electrophilic addition reaction. Negative chemical ionization reactions mainly include electron trapping reaction and negative ion addition reaction.
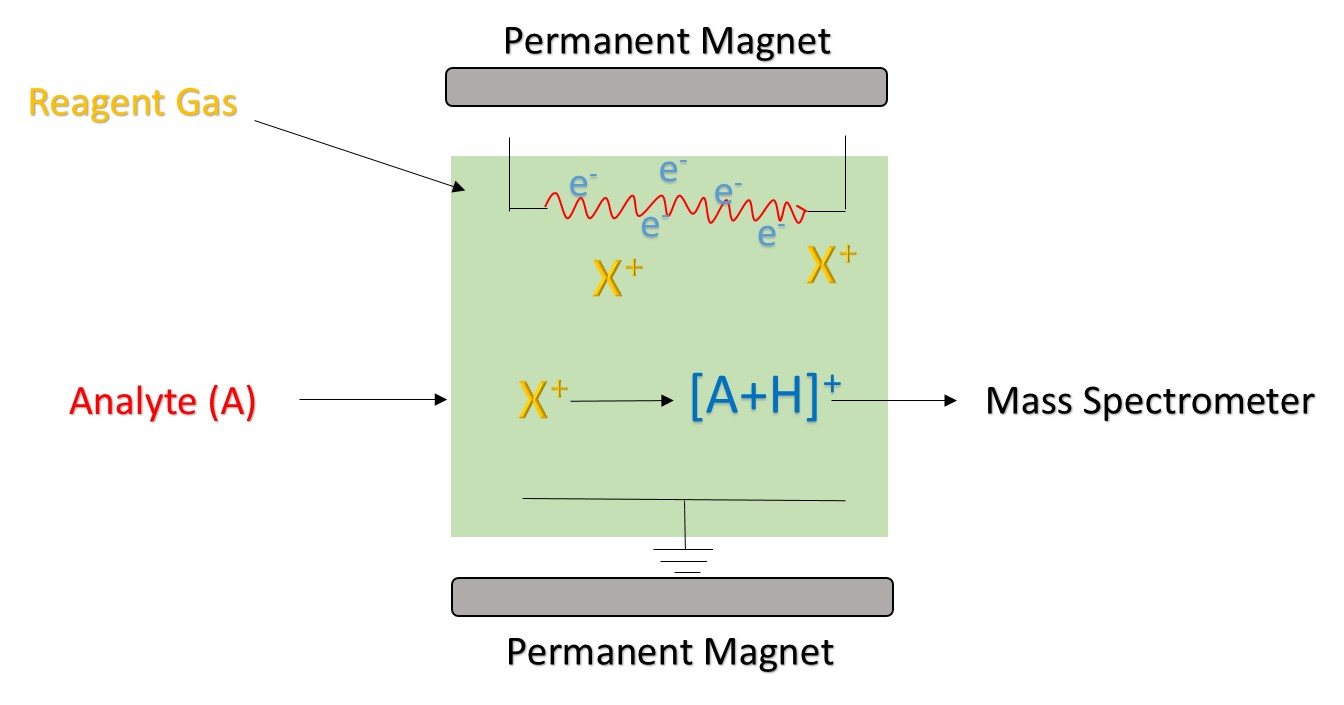 Figure 1. The principle of chemical ionization
Figure 1. The principle of chemical ionization
Advantages and Disadvantages of CI
The resolution of the CI spectrum is simple and the exact molecular weight of the analyte can be obtained. The product obtained by CI has few fragments, and its products are mainly molecules and ions of the analyte. The selectivity of CI can be easily increased by selecting an appropriate reagent ion. For example, the reagent ion H3O+ reacts only with an organic substance having a proton affinity greater than H3O+. In addition, CI has high sensitivity and fast response (15s). However, if the reactive ions are impure and a variety of chemical ionization reactions will occur at the same time, the mass spectrometry becomes difficult.
Examples of CI Applications
At present, CI is widely used as an ion source in mass spectrometry systems to detect various trace substances. CI can be used for the detection of substances such as trace gases in the atmosphere, pesticide residues in vegetables and fruits, melamine in milk powder, plasticizers and herbicides in soil. In addition, CI can also be used for material identification, such as identifying the quality of tea.
- Measurement of volatile organic compounds (VOC)
In the measurement of VOC by CI, H3O+ is generally used as a reagent ion. H3O+ does not react with most substances in the air such as O2, N2, CO2, etc. On the other hand, most of the proton transfer reactions are non-dissociated, so the product ions are single, which makes the analysis of the results simple. The principle of measuring VOC using H3O+ as reagent ion is shown in equation (3). The final product was resolved by proton transfer reaction mass spectrometry.
- Determination of melamine in milk powder
In the measurement of melamine by CI, N2 is used as a reagent gas. Under the action of high voltage, N2 and H2O undergo complex physicochemical reactions to generate reagent ions H3O+. The reaction process is shown in formula (3-6). H3O+ reacts with melamine in the milk powder, and the reaction principle is shown in formula (2).
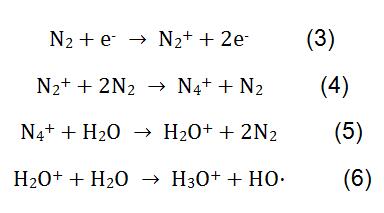
- Identification of the tea quality
Different types of tea can be identified by measuring the chemical substances on the surface of tea leaves with CI. H3O+ reacts with the surface of the tea with butanol, geraniol, caffeine and other substances, and the reaction products form different mass spectra on the mass spectrometer. The content of the same substance in different varieties of tea is different, so the mass spectrum formed by CI detection is different. The mass spectrum can reflect the chemical fingerprint characteristics of the tea to some extent. Therefore, CI has important practical application value for the rapid identification and quality analysis of tea.
Chemical ionization is widely used in mass spectrometry due to its strong selectivity and high sensitivity. Chemical ionization mass spectrometry (CIMS) not only enables tandem mass spectrometry identification of substances in complex samples, but also obtains chemical fingerprint data for samples for chemometric analysis. The application of CIMS has a positive effect on promoting the development of food, medicine, environmental protection, agriculture and other industries.
We have briefly introduced the CI, a type of ionization method, which can help you understand more about mass spectrometry. At Creative Proteomics, we have developed the professional mass spectrometry platform, which contains state-of-the-art instruments. By using mass spectrometry, Creative Proteomics can provide different services to meet various requirements, including:
References
- Fales H M; et al. Comparison of mass spectra of some biologically important compounds as obtained by various ionization techniques. Analytical Chemistry, 1975, 47(2):207-219.
- Lindinger W; et al. On-line monitoring of volatile organic compounds at pptv levels by means of proton-transfer-reaction mass spectrometry (PTR-MS) medical applications, food control and environmental research. International Journal of Mass Spectrometry & ion Processes, 1998, 173(3):191-241.
- Chen H; et al. Surface desorption atmospheric pressure chemical ionization mass spectrometry for direct ambient sample analysis without toxic chemical contamination. Journal of Mass Spectrometry, 2010, 42(8):1045-1056.
- Yang S; et al. Detection of Melamine in Milk Products by Surface Desorption Atmospheric Pressure Chemical Ionization Mass Spectrometry. Analytical Chemistry, 2009, 81(7):2426.






