- Service Details
- FAQ
What is Atmospheric Pressure Photoionization Ionization?
Atmospheric Pressure Photoionization Ionization (APPI) is a soft ionization technique used in mass spectrometry, particularly in conjunction with liquid chromatography (LC-MS). It is designed to ionize molecules in the gas phase at atmospheric pressure, making it especially useful for analyzing weakly polar and non-polar compounds that are often challenging to ionize using other techniques like Electrospray Ionization (ESI) or Atmospheric Pressure Chemical Ionization (APCI).
Principle of APPI
In APPI, the solvent and sample from the liquid chromatography first form a gaseous analyte (M), which is ionized to interact with photons emitted by the light source, and the ions are introduced into the mass spectrometer for analysis. During this process, both the analyte and the solvent may be excited, and the analyte may also acquire protons from the protic solvent:
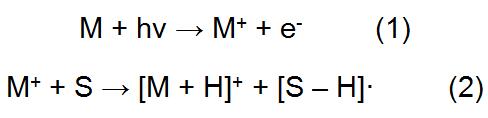
Wherein M represents an analyte and S represents a solvent. Since both the solvent and the analyte are likely to be ionized, the emission energy of the ultraviolet light source should be greater than the ionization potential of the analyte and less than the ionization potential of the air component and the solvent, which can reduce the amount of impurity ions entering the mass spectrometer. The APPI light source can be derived from an argon lamp, a xenon lamp, a xenon lamp, or the like. Among them, xenon lamps are used most frequently, and the red light energy emitted by xenon lamps is between common solvents and most compounds.
The method in which the analyte in the formula (1) is directly ionized is called direct APPI. In addition, the amount of ions generated by direct photoionization of some analytes is small, so it is necessary to add one or several suitable substances to help the analyte to form ions in a large amount. The medium that can help the analyte to ionize is called a dopant. The typical dopant involved in the APPI process is as follows (D represents the dopant):
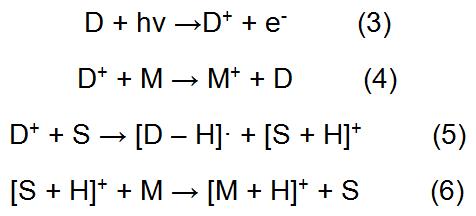
The APPI process in which the dopant is involved is referred to as dopant-assisted APPI (DA-APPI). As a medium for photoionization, the dopant helps the analyte to form ions mainly by exchanging charge or proton transfer. Initially, the dopant is ionized after receiving photons. The ionized dopant ions (D+) can exchange charge directly with the analyte to form M+. On the other hand, the D+ can also transfer the obtained positive charge to the solvent, and then the protons are transferred to the analyte by the solvent, thereby forming enough [M + H]+ to enter the mass spectrometer. It can be known from the DA-APPI process that the two basic conditions as a dopant are that the ionization potential is lower than the energy of the photon emitted by the light source and the proton affinity is low.
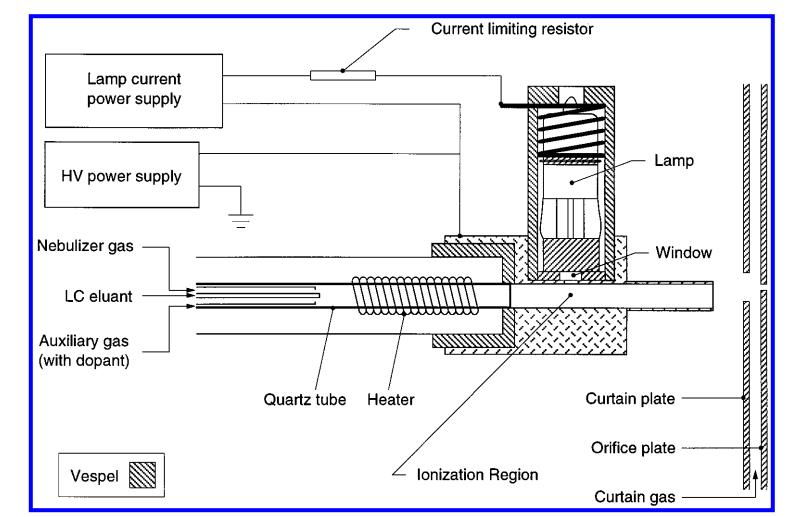 Figure 1. Schematic of the APPI ion source, including the heated nebulizer probe, photoionization lamp, and lamp mounting bracket (Robb D B, 2000).
Figure 1. Schematic of the APPI ion source, including the heated nebulizer probe, photoionization lamp, and lamp mounting bracket (Robb D B, 2000).
Advantages of the APPI Platform
- Broad Applicability: Efficiently ionizes both polar and non-polar small molecules, allowing for comprehensive analysis within a single LC-MS run.
- Enhanced Sensitivity and Specificity: Minimizes matrix effects and ion suppression, resulting in cleaner data and improved analyte recovery rates.
- Wide Dynamic Range: Offers a dynamic linear range of five orders of magnitude, making it ideal for accurate quantitative analysis.
- Simplified Sample Preparation: Reduces the need for extensive sample purification, saving time and streamlining the analysis process.
APPI Platform Services at Creative Proteomics
At Creative Proteomics, we leverage our advanced APPI (Atmospheric Pressure Photoionization Ionization) platform to offer cutting-edge analytical services tailored to your research and industrial needs. Our APPI services are designed to provide high-quality, reliable data for a diverse range of applications. Here's how we can support your analytical goals:
Comprehensive Proteomics Services
Our APPI platform is adept at analyzing complex protein samples. We provide detailed proteomics services that include:
- Protein Identification and Protein Quantification: Accurate identification and quantification of proteins from complex biological samples.
- Peptide Mapping: Detailed mapping of peptide sequences for in-depth protein characterization.
- Post-Translational Modifications: Analysis of modifications such as phosphorylation, glycosylation, and ubiquitination.
- Biomarker Discovery: Identification of potential biomarkers for disease and therapeutic targets.
Metabolomics Services
Unlock valuable insights into metabolic processes with our metabolomics services:
- Metabolite Profiling: Comprehensive profiling of metabolites to understand metabolic pathways and identify biomarkers.
- Quantitative Analysis: Precise quantification of metabolites for accurate data interpretation.
- Metabolic Pathway Analysis: Mapping of metabolic pathways to reveal biological functions and mechanisms.
In-Depth Glycomics Services
Our glycomics services focus on the detailed analysis of glycan structures:
- Glycan Profiling: Identification and quantification of glycan structures from various biological samples.
- Glycosylation Analysis: Study of glycosylation patterns and their implications in protein function and disease.
- Structural Elucidation: Detailed analysis of glycan structures to understand their role in biological systems.
Sample Requirements for APPI
| Sample Type | Details | Recommended Volume |
|---|---|---|
| Plasma/Serum Samples | Includes human or animal plasma or serum used for proteomics and metabolomics studies. Ensure proper filtration. | 1-10 microliters |
| Urine Samples | Human or animal urine, prepared in suitable solvents for analysis. Filtration may be required to remove particulates. | 1-10 microliters |
| Tissue Homogenates | Extracts from tissue samples (e.g., liver, brain) prepared in compatible solvents. Ensure samples are filtered. | 1-10 microliters |
| Cell Lysates | Lysates from cultured cells, prepared in solvents compatible with APPI. Ensure complete solubilization and filtration. | 1-10 microliters |
| Drug Solutions | Solutions of pharmaceutical drugs or drug metabolites in solvents suitable for APPI. Must be free from impurities that could affect ionization. | 1-10 microliters |
| Environmental Water Samples | Includes water samples from various environmental sources (e.g., rivers, lakes) spiked with contaminants if necessary. Ensure proper filtration. | 1-10 microliters |
| Food and Beverage Extracts | Extracts from food items or beverages, prepared in solvents compatible with APPI. Ensure samples are clear of solid residues. | 1-10 microliters |
| Biological Fluids (e.g., Saliva) | Includes saliva samples for biomarker analysis. Must be prepared in solvents suitable for APPI and filtered. | 1-10 microliters |
Applications of the APPI Platform
Environmental Pollutant Detection
The impact of human activity has led to an increasing prevalence of pollutants in our environment, contaminating water, air, soil, and biota. LC-APPI-MS plays a critical role in detecting trace levels of pharmaceutical residues, pesticides, fungicides, food additives, and industrial chemicals in environmental samples. For instance, using toluene as a dopant, LC-APPI-MS can detect pharmaceutical residues such as acetaminophen, caffeine, and naproxen in water at detection limits ranging from 0.3 ng/L to 15 ng/L.
Detection of Harmful Substances in Human Samples
APPI also proves indispensable in clinical and biomedical research. It is highly effective in analyzing harmful substances in human blood, offering low detection limits and rapid analysis times. For example, the detection of toxicants like paraquat and diquat in blood samples treated with formic acid and acetonitrile yields intra-day recovery rates of 99.0% and 91.9%, respectively, with relative standard deviations of less than 6.5%.
Detection of Antibiotic Residues in Food
The presence of antibiotics in the food supply is a growing concern due to their impact on environmental microorganisms, animal and plant growth, and human health. APPI is particularly well-suited for the detection of antibiotic residues, such as chloramphenicol, in food products like fish. Detection limits for chloramphenicol in carp and flounder are as low as 0.27 ng/g and 0.10 ng/g, respectively. Additionally, APPI can detect 18 types of aminobenzene sulfonamides in honey, with detection limits between 0.4 and 4.5 μg/kg, offering superior sensitivity compared to ESI and APCI sources.
Why is the recommended sample volume for APPI 1-10 microliters?
The recommended volume range of 1-10 microliters for APPI analysis is based on several factors:
- Sensitivity: APPI is sensitive enough to detect compounds in low concentrations, and volumes within this range typically provide sufficient analyte concentration for accurate detection without overloading the system.
- Ionization Efficiency: A smaller volume helps maintain optimal ionization conditions. Excessive volumes may lead to issues such as ion suppression or saturation of the detector.
- Instrument Specifications: Most APPI-equipped mass spectrometers are designed to handle sample volumes within this range, ensuring that the ionization and detection processes are efficient and reliable.
For specific analyses or instruments, this volume range may vary. It's essential to consult with the APPI platform provider for guidance tailored to your specific needs.
How should samples be prepared before analysis to ensure accurate results?
Proper sample preparation is crucial for accurate APPI analysis:
- Filtering: Samples should be filtered or centrifuged to remove particulates that could clog the system or interfere with ionization.
- Dissolution: Ensure that solid samples are fully dissolved in a suitable solvent. Incomplete dissolution can lead to inaccurate results and poor ionization.
- Solvent Compatibility: Use solvents that are compatible with APPI to avoid interference. Common solvents include acetonitrile, methanol, and water, but the choice depends on the sample type and the analytes of interest.
- Avoid Contamination: Handle samples with clean equipment to prevent contamination that could skew results. Use appropriate containers and tools to maintain sample integrity.
Adhering to these preparation steps helps achieve high-quality data and reliable results from the APPI analysis.
What are matrix effects, and how can they be minimized in APPI analysis?
Matrix effects occur when components in the sample matrix interfere with the ionization of the analytes. They can lead to inaccurate quantification and reduced sensitivity. To minimize matrix effects:
- Sample Purification: Use techniques like filtration, centrifugation, or solid-phase extraction to clean up the sample and reduce matrix interference.
- Use Internal Standards: Adding internal standards can help correct for matrix effects and improve quantification accuracy by compensating for any variations in ionization.
- Optimize Solvent Choice: Select solvents that reduce matrix effects and ensure compatibility with the APPI process. Avoid solvents that might cause ion suppression or enhancement.
By addressing matrix effects, you can enhance the accuracy and reliability of your APPI analysis.
Can APPI be used for quantitative analysis, and what are its limitations?
Yes, APPI is suitable for quantitative analysis, particularly for non-polar and weakly polar compounds. Its advantages include:
- Wide Dynamic Range: APPI provides a broad dynamic range, making it useful for quantifying compounds over a large concentration range.
- Minimal Matrix Effects: APPI typically reduces matrix effects compared to other ionization techniques, aiding in accurate quantification.
However, APPI also has limitations:
- Lower Sensitivity for Some Compounds: APPI may be less sensitive than other ionization techniques like ESI (Electrospray Ionization) for certain analytes, particularly those that are highly polar.
- Thermal Instability: APPI is not ideal for compounds with poor thermal stability, as the ionization process involves exposure to UV light and heat.
How does APPI compare to other ionization techniques like ESI and APCI?
APPI, ESI (Electrospray Ionization), and APCI (Atmospheric Pressure Chemical Ionization) are distinct ionization techniques used in mass spectrometry, each with unique advantages:
- APPI: Best suited for weakly polar and non-polar compounds. It utilizes UV light to ionize samples, which is advantageous for analyzing compounds that are challenging for ESI or APCI. APPI is particularly effective for analyzing complex matrices with minimal ion suppression.
- ESI: Ideal for highly polar and ionic compounds. It produces ions in solution by applying a high voltage, making it excellent for biomolecules such as proteins and peptides. ESI is highly sensitive but may struggle with non-polar compounds.
- APCI: Suitable for semi-polar and non-polar compounds. It uses a corona discharge to ionize the sample, making it effective for compounds with moderate polarity. APCI is less sensitive than ESI but can handle a broader range of compounds than APPI.
The choice between these techniques depends on the nature of the sample and the specific analytical requirements.
What is the role of dopants in APPI, and how do they affect the analysis?
Dopants are substances added to the sample to enhance ionization in APPI. They play a crucial role in improving the sensitivity and efficiency of the ionization process:
- Function: Dopants help generate more ions by either transferring protons or exchanging charges with the analytes. They can significantly increase the ionization of compounds that otherwise produce low signal intensities.
- Selection: The choice of dopant depends on the ionization potential of the analyte and the light source used. Common dopants include toluene, chlorobenzene, and anthracene.
- Effect on Analysis: Proper selection and concentration of dopants can improve detection limits and quantitative accuracy. However, excessive dopant concentration may lead to background noise or interference.
Understanding how to use dopants effectively can enhance the performance of APPI and ensure accurate results.
What are the common issues encountered with APPI, and how can they be resolved?
Low Sensitivity: If the sensitivity is lower than expected, ensure that the sample concentration is adequate and that the solvent and dopant are compatible. Verify the lamp intensity and alignment as well.
High Background Noise: This can result from impurities or improper sample preparation. Filter samples thoroughly and ensure that the system is clean and free of contaminants.
Ion Suppression: This occurs when matrix components interfere with ionization. Use internal standards to correct for suppression effects and consider sample cleanup methods to reduce matrix interference.
Thermal Instability of Samples: If the sample degrades during ionization, consider using a lower energy UV source or optimizing the sample preparation to minimize thermal exposure.
How can I ensure reproducibility in APPI experiments?
- Consistent Sample Preparation: Use standardized procedures for sample extraction, dissolution, and filtration to minimize variability.
- Calibration and Maintenance: Regularly calibrate the mass spectrometer and maintain the APPI source to ensure consistent performance.
- Use of Internal Standards: Include internal standards in your samples to account for any variations in ionization efficiency and improve quantification accuracy.
- Control Experimental Conditions: Keep experimental conditions, such as temperature and solvent composition, consistent across different runs to reduce variability.
What types of sample contaminants can affect APPI, and how should they be managed?
Sample contaminants that can affect APPI include:
Particulates: Solid particles can clog the system or interfere with ionization. Filter or centrifuge samples to remove particulates before analysis.
Chemical Impurities: Residual solvents or other chemicals may affect ionization. Use high-purity solvents and ensure that samples are free from contaminants.
Matrix Components: Components from the sample matrix may cause ion suppression or enhancement. Minimize matrix effects through sample purification and by using internal standards.
How should samples be stored before analysis to preserve their integrity?
Proper storage of samples is crucial to preserve their integrity before analysis:
- Short-Term Storage: Store samples in a cool, dry place if they will be analyzed within a short period (hours to a few days).
- Long-Term Storage: For longer storage, freeze samples at -20°C or lower. Avoid repeated freeze-thaw cycles as they can degrade samples.
- Avoid Light Exposure: Some compounds may degrade upon exposure to light, so store samples in dark containers if necessary.










