- Service Details
- Results
What Is Western Blot
The transfer or "blotting" of electrophoretic separated proteins from the gel matrix to a membrane (typically nitrocellulose or PVDF) followed by subsequent antibody-based detection on the surface of the membrane is called Western Blot or Immunoblot. Creative Proteomics provides western blotting analysis for the detection of a specific target protein out of a complex protein mixture, e. g. tissue homogenate or cell extract, using highly selective and sensitive antibody-antigen interactions. The resulting data allow both qualitative and semi-quantitative analysis of the protein of interest.
The Western Blot is a widely used analytical technique for the detection of specific proteins in a sample of tissue homogenate or extract. It uses gel electrophoresis to separate native proteins by 3-D structure, or denatured proteins by the length of the polypeptide. The separated proteins are then transferred to a membrane (typically nitrocellulose or PVDF), where they are stained with antibodies specific to the target protein. Western Blot is widely used in the fields of molecular biology, biochemistry, immune genetics and other molecular biology disciplines. Blotting is the transfer of macromolecules on immobilizing membranes for specific and sensitive detection.
What Our Western Blot Detection Service offers
Protein Extraction: Extraction of protein samples from human or animal cells, tissues, bacteria, etc.
Protein Quantification: Semi-quantitative analysis of proteins in the samples.
Western Blot Detection
(1) Electrophoresis: Separation of protein samples using Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE).
(2) Wet Transfer.
(3) Hybridization: Identification of specific proteins on the membrane using antibodies.
(4) Visualization: Fluorescence staining, black bands on a white background image (green bands on a black background fluorescence image).
Internal Reference Protein Detection
Pre-experiment and Formal Experiment Services.
Advantages of Western Blot Detection Service
Utilization of different protein concentration determination methods (Lowry or BCA) for various protein samples.
Ensuring experiment quality through rigorous QC (Quality Control): Accurate quantification using internal references, with quantification values showing less than 8% variation across multiple experiments on the same sample.
Ensuring result accuracy.
Comprehensive technical services, ranging from gene synthesis, cell transfection, vector construction, protein expression and purification, to protein functional studies.
Years of antibody research experience, with extensive knowledge in antibody selection to mitigate false-positive results at the source.
Sample Requirements
Cells: >1x10^7; Tissues: >30mg; Bacterial Wet Weight: >1mg, etc.
Sample protein concentration: >1mg/ml; Protein amount: >200ug/sample.
It is recommended to provide positive control samples for the target protein (pure protein, cells or tissues with positive expression, commercially available positive control products, etc.) as positive controls.
Applications of Western Blot
Widely used for protein expression level detection.
Identification of specific proteins; can also be used for quantitative and semi-quantitative analysis of target proteins.
Suitable for subsequent analysis of protein-protein, protein-DNA, and protein-RNA interactions.
Advantages of Western Blot
Requires convenient equipment, low cost, and flexible usage.
Simple operation, generally stable results, and reliable positive outcomes.
Result analysis is comparatively straightforward compared to mass spectrometry.
Issues and Strategies Frequently Encountered in Western Blot Experiments
| Experimental Issue | Possible Reasons | Recommended Solutions |
|---|---|---|
| Absence of Target Band | Low expression of target protein | * Increase sample loading, concentrate target protein, use more sensitive reagents |
| Insufficient antibody potency | * Use freshly prepared primary antibody, avoid repeated freeze-thaw cycles; increase primary or secondary antibody concentration, extend incubation time | |
| Low transmembrane efficiency | * Choose appropriate transfer method, improve transfer efficiency | |
| Non-parallel, Curved Bands | Rapid electrophoretic migration or high migration temperature | * Adjust electrophoresis settings such as pH, voltage, etc., decrease ambient temperature during electrophoresis |
| High Background | Inappropriate blocking conditions | * Extend blocking time or change blocking agent |
| High antibody concentration | * Reduce primary/secondary antibody concentration and extend incubation time | |
| Cross-reaction between antibody and blocking agent | * Use additives like Tween 20 and detergents to minimize cross-reaction |
Principle And Workflow of Western Blot
In Western Blot analysis, the proteins of the sample are first separated in gel electrophoresis by isoelectric point (pI), molecular weight, electric charge, or a combination of these factors using various methods such as SDS-PAGE and IEF. Usually SDS-PAGE is employed for the separation, because all the proteins are solubilized and migrate in the same direction, and the epitopes are easier accessible due to the denaturing effect of SDS.
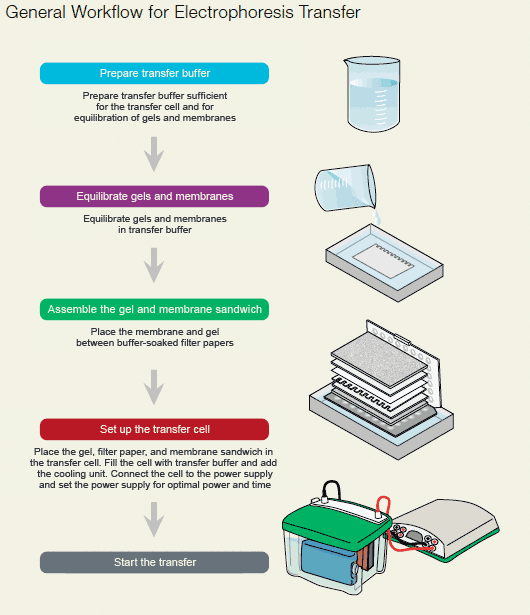
After the gel electrophoresis analysis, the proteins are moved from within the gel onto a membrane made of nitrocellulose or polyvinylidenedifluoride (PVDF) to make them accessible for antibody detection. PVDF membranes have a higher binding capacity for proteins than nitrocellulose, but nitrocellulose binds small proteins better. The primary method for transferring the proteins is called electroblotting which uses an electric current to pull proteins from the gel into the PVDF or nitrocellulose membrane. There are several ways to perform the electrophoretic transfer: tank blotting, semi-dry blotting and semi-wet blotting. All three have in common is that the gel and the membrane form a sandwich with a stack of filter papers on both sides. It should be noted that, if isoelectric focusing is used for the separation of the proteins, it is more efficient to transfer the proteins by diffusion with pressure blotting. During electroblotting, the proteins move from within the gel onto the membrane while maintaining the organization they had within the gel.
After the blotting process, which can take about an hour (semidry blotting) to overnight (tank blotting), the proteins are exposed on a thin surface layer for antibody detection. Besides, the free binding sites of the membrane are blocked with a protein mixture, which will not interfere with the subsequent probing with an antibody. The proteins are first detected by the primary antibody, and then detected with a secondary antibody, which recognizes this particular primary antibody. The secondary antibody is conjugated with a set of specific molecules, which can be easily detected with a subsequent development procedure with high sensitivity.
The most sensitive detection methods are using enhanced chemiluminescence (ECL): the antibody–horseradish conjugate recognizes the primary antibody; the substrate reaction is coupled with a secondary reaction which causes chemiluminescent light emission for a certain time period. This light signal is accumulated by exposing the membrane on an X-ray film, or by placing it into an absolutely dark cabinet where the signal is recorded with a sensitive CCD camera. With a special variant of ECL, down to 1 pg of a protein band is detectable.
Western Blotting could be used to follow protein phosphorylation. An antibody that binds to all the isoforms of a poly-phosphorylated protein will show a 'beads-on-a-string" look on a 2D gel. Western Blotting is also useful for identifying known and unknown proteins in complexes brought down by co-immunoprecipitation. Once proteins of interest are located on a Western blot, corresponding spots can be cut from a duplicate Coomassie blue-stained gel and identified by MS.








