- Service Details
- Demo
- Case Study
- FAQ
What is Purine?
A purine is a heterocyclic aromatic organic compound that consists of a pyrimidine ring fused to an imidazole ring. Purine gives its name to the wider class of molecules, purines, which include substituted purines and their tautomers, are the most widely occurring nitrogen-containing heterocycle in nature. Purine is water soluble. Purines are found in high concentration in meat and meat products, especially internal organs such as liver and kidney.
Purines and pyrimidines make up the two groups of nitrogenous bases, including the two groups of nucleotide bases. Two of the four deoxyribonucleotides (deoxyadenosine and deoxyguanosine) and two of the four ribonucleotides (adenosine, or AMP, and guanosine, or GMP), the respective building blocks of DNA and RNA, are purines. In order to form DNA and RNA, both purines and pyrimidines are needed by the cell in approximately equal quantities. Both purine and pyrimidine are self-inhibiting and activating. When purines are formed, they inhibit the enzymes required for more purine formation. This self-inhibition occurs as they also activate the enzymes needed for pyrimidine formation. Pyrimidine simultaneously self-inhibits and activates purine in similar manner. Because of this, there is nearly an equal amount of both substances in the cell at all times.
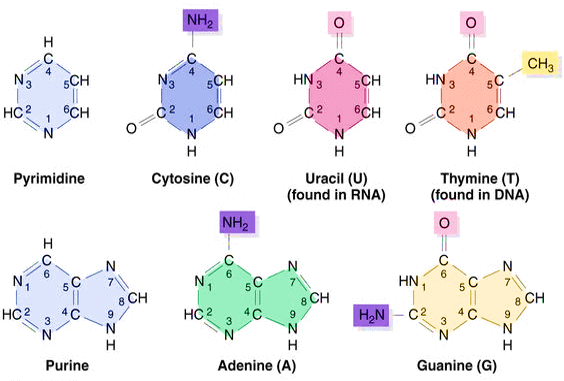 Figure 1. Purines and pyrimidine bases.
Figure 1. Purines and pyrimidine bases.
Many organisms have metabolic pathways to synthesize and break down purines. Purines serve as the building blocks of DNA and RNA, as well as key energy substrates and cofactors. Their metabolites, including ATP, GTP, NADH, and coenzyme A, are crucial for various biological processes like energy metabolism, signal transduction, and enzyme regulation. Accumulation of modified purine nucleotides is defective to various cellular processes, especially those involving DNA and RNA. To be viable, organisms possess a number of (deoxy)purine phosphohydrolases, which hydrolyze these purine derivatives removing them from the active NTP and dNTP pools. Deamination of purine bases can result in accumulation of such nucleotides as ITP, dITP, XTP and dXTP. Defects in enzymes that control purine production and breakdown can severely alter a cell's DNA sequences, which may explain why people who carry certain genetic variants of purine metabolic enzymes have a higher risk for some types of cancer. Higher levels of meat and seafood consumption are associated with an increased risk of gout, whereas a higher level of consumption of dairy products is associated with a decreased risk. Moderate intake of purine-rich vegetables or protein is not associated with an increased risk of gout.
 Diagram of the purine catabolism pathway.
Diagram of the purine catabolism pathway.
At Creative Proteomics, we offer an extensive suite of purines analysis services designed to meet the diverse needs of both academic and industrial researchers.
Analytical Projects Provided by Creative Proteomics
Purine Identification and Quantification
Utilizing advanced High-Performance Liquid Chromatography (HPLC) and Mass Spectrometry (MS) techniques, we accurately identify and quantify purine compounds in various sample types. This service includes:
- Detection of Purine Metabolites: Comprehensive analysis of purine metabolites, including adenosine, guanosine, hypoxanthine, xanthine, and uric acid.
- Quantitative Analysis: Precise measurement of purine concentrations, reported in µM or µg/mg, ensuring data accuracy and reproducibility.
Nucleotide Profiling
We offer detailed profiling of nucleotides, which are the building blocks of DNA and RNA. This service involves:
- Ribonucleotide Analysis: Identification and quantification of ribonucleotides such as ATP, GTP, AMP, and GMP.
- Deoxyribonucleotide Analysis: Analysis of deoxyribonucleotides including dATP, dGTP, dAMP, and dGMP.
Pathway Analysis of Purine Metabolism
Understanding the metabolic pathways of purines is crucial for various biological studies. Our pathway analysis includes:
- Enzyme Activity Assays: Measurement of the activity levels of key enzymes involved in purine metabolism, such as adenylate kinase, guanylate kinase, and xanthine oxidase.
- Metabolic Flux Analysis: Examination of the flux through purine metabolic pathways to understand the dynamic changes and regulatory mechanisms.
Purine Derivative Analysis
We analyze purine derivatives that play significant roles in cellular processes. This service covers:
- Modified Nucleotides: Detection and quantification of modified purine nucleotides such as inosine monophosphate (IMP), xanthosine monophosphate (XMP), and other derivatives.
- Nucleotide Analogues: Analysis of synthetic nucleotide analogues used in therapeutic and research applications.
Purine Receptor Analysis
Purine receptors are crucial for many physiological functions. Our analysis includes:
- P1 and P2 Receptors: Detailed study of purine receptors, including adenosine receptors (P1) and P2 receptors (P2X and P2Y subtypes).
- Receptor Binding Assays: Investigation of the binding affinities and interaction dynamics between purines and their receptors.
Custom Purines Analysis
We offer tailored purines analysis services to meet specific research requirements. Custom projects can include:
- Novel Purine Compounds: Identification and characterization of novel purine compounds.
- Targeted Metabolomics: Focused analysis of specific purine-related pathways or metabolites based on research needs.

Brochures
Metabolomics Services
We provide unbiased non-targeted metabolomics and precise targeted metabolomics services to unravel the secrets of biological processes.
Our untargeted approach identifies and screens for differential metabolites, which are confirmed by standard methods. Follow-up targeted metabolomics studies validate important findings and support biomarker development.
Download our brochure to learn more about our solutions.
Analytical Techniques for Purines
High-Performance Liquid Chromatography (HPLC)
High-Performance Liquid Chromatography (HPLC) is a cornerstone of our purines analysis, offering high resolution and sensitivity. Key features of our HPLC methodology include:
- Separation Efficiency: Superior separation of purine compounds from complex biological matrices.
- Sensitivity: Detection of purine metabolites at low concentrations, ensuring accurate quantification.
- Reproducibility: Consistent results across multiple analyses due to precise control of chromatographic conditions.
Mass Spectrometry (MS)
Mass Spectrometry (MS) provides detailed molecular characterization and accurate quantification of purines. Our MS techniques include:
- Electrospray Ionization (ESI): A soft ionization method that preserves the integrity of purine molecules for accurate mass analysis.
- Time-of-Flight (TOF): High-resolution mass spectrometry that provides precise molecular weight determination.
- Tandem Mass Spectrometry (MS/MS): Enhanced structural elucidation through fragmentation and analysis of purine compounds.
Ultra-High-Performance Liquid Chromatography (UHPLC)
Ultra-High-Performance Liquid Chromatography (UHPLC) is employed for even higher resolution and faster analysis times. Advantages of UHPLC include:
- Increased Speed: Faster run times compared to traditional HPLC, improving throughput.
- Higher Resolution: Better separation of closely related purine compounds.
- Improved Sensitivity: Enhanced detection limits for trace level analysis.
Nuclear Magnetic Resonance (NMR) Spectroscopy
Nuclear Magnetic Resonance (NMR) Spectroscopy is used for structural elucidation and confirmation of purine compounds. Benefits of NMR include:
- Structural Insights: Detailed information on the molecular structure and conformation of purines.
- Non-Destructive: Preservation of sample integrity for subsequent analyses.
- Quantitative Analysis: Accurate quantification of purines in complex mixtures.
Liquid Chromatography-Mass Spectrometry (LC-MS)
Liquid Chromatography-Mass Spectrometry (LC-MS) combines the separation power of liquid chromatography with the detection capabilities of mass spectrometry. Highlights of LC-MS include:
- Comprehensive Analysis: Simultaneous separation and identification of multiple purine compounds.
- Enhanced Sensitivity: Superior detection limits for low-abundance purines.
- Versatility: Applicable to a wide range of sample types and purine derivatives.
Gas Chromatography-Mass Spectrometry (GC-MS)
Gas Chromatography-Mass Spectrometry (GC-MS) is employed for the analysis of volatile purine derivatives. Key aspects of GC-MS include:
- Volatility Analysis: Effective for purines that can be derivatized into volatile forms.
- High Sensitivity: Detection of purine compounds at trace levels.
- Quantitative Accuracy: Precise quantification of purine concentrations in complex matrices.
Sample Requirements for Purines Analysis
For optimal results in purines analysis, we recommend specific sample types and quantities. Below is a detailed guide on sample requirements:
| Sample Type | Recommended Amount | Remark |
|---|---|---|
| Animal Tissue | 100-200 mg | Store at -80℃ and send with enough dry ice |
| Plant Tissue | 100-200 mg | |
| Plasma/Serum | >100 μL | |
| Urine | 200-500 μL | |
| Saliva, Amniotic fluid, Bile, Tears, etc. | >200 μL | |
| Cells | >1*107 | |
| Culture Supernatant | >2 mL | |
| Wastewater/Culture Medium | >2 mL | |
| Microbial Culture | >2 mL | |
| Feces/Intestinal Contents | 100-200 mg | |
| Soil Sample | >1 g | |
| Swab | 2 |
Delivery
- Experiment Procedure: A step-by-step outline of the analytical procedures employed.
- Instrument Parameters: Detailed information on the settings and conditions of the instruments used.
- Analytes Report: Concentrations of analytes are reported in µM or µg/mg (tissue), with Coefficients of Variation (CVs) generally below 10%.
- Analyte Information: Detailed data on each analyte, including name, abbreviation, formula, molecular weight, and CAS number.
Concentrations of the detected compounds were calculated with internal-standard calibration by interpolating the constructed linear calibration curves of individual compounds within an appropriate concentration range for each compound.
| Sample_C(µmol/g or ppm) | Adenine | Adenosine | Cytidine | Cytosine |
|---|---|---|---|---|
| 01_S11 | 0.010137697 | 13.19207811 | 0.07989675 | 0.025798554 |
| 02_S12 | 0.006172711 | 13.08010022 | 0.071507282 | 0.024001392 |
| 03_S21 | 0.005079556 | 15.49480016 | 0.066231695 | 0.035768753 |
| 04_S22 | 0.004049685 | 14.58872029 | 0.056179632 | 0.035162897 |
| 05_S31 | 0.178592086 | 14.92748755 | 0.061898612 | 0.043892462 |
| 06_S32 | 0.145836273 | 16.70362739 | 0.047692858 | 0.04157569 |
| 07_S41 | 0.153798631 | 15.19871775 | 0.063177346 | 0.041044176 |
| 08_S42 | 0.106759819 | 14.66986091 | 0.060294108 | 0.0414638 |
| 09_S51 | 0.532269225 | 12.3470456 | 0.078734577 | 0.038760706 |
| 10_S52 | 0.533387532 | 14.68101141 | 0.013787334 | 0.034976939 |
| QC_01 | 0.009877496 | 13.19207811 | 0.079765046 | 0.025482708 |
| QC_02 | 0.010137697 | 13.57015856 | 0.07989675 | 0.025798554 |
| QC_03 | 0.009793479 | 13.30295421 | 0.079674113 | 0.027084818 |
| CV% | 1.8 | 1.5 | 0.1 | 3.2 |
Comprehensive quantification of purine and pyrimidine metabolism in Alzheimer's disease postmortem cerebrospinal fluid by LC–MS/MS with metal-free column
Journal: Biomedical Chromatography
Published: 2019
Background
Alzheimer's disease (AD) is a neurodegenerative disorder characterized by progressive cognitive decline and behavioral disturbances in the elderly. Pathologically, it involves neurofibrillary tangles of tau protein and amyloid β plaques. Beyond these, mitochondrial dysfunction, neuroinflammation, and neurotransmitter disruptions are implicated in its progression. Purine and pyrimidine metabolism, critical for energy transfer and cellular regulation, have been linked to AD pathology.
Technical Methods
The study employed liquid chromatography with tandem mass spectrometry (LC–MS/MS) for comprehensive analysis of 40 nucleobases, nucleosides, nucleotides, and phosphorylated metabolites involved in purine and pyrimidine metabolic pathways, typical of metabolomics studies. A metal-free PEEK column was utilized to overcome chromatographic challenges associated with interactions between phosphorylated residues and metal surfaces, which typically lead to peak tailing and reduced sensitivity.
The optimized LC–MS/MS method utilized a mobile phase of pH 7 with 10 mM ammonium formate to enhance stability and sensitivity, essential for metabolite detection in complex biological samples. Injection volume was set at 2 μl to ensure optimal peak shape and avoid double-peaking issues observed at higher volumes. Gradient elution conditions were tailored to achieve efficient separation and cleaning of the column, crucial for maintaining consistent performance and minimizing carryover in metabolomics analyses.
Results
Analytical performance validation demonstrated the method's robustness: calibration curves exhibited excellent linearity (r² > 0.9959) across a concentration range of 5 nM to 0.1 mM for all compounds. The method achieved low limits of detection (LOD) ranging from 0.5 to 250 nM and limits of quantification (LOQ) ranging from 1 to 500 nM, surpassing those reported for ion-pairing LC–MS/MS methods (Laourdakis, 2014). Recovery rates for most compounds ranged from 70.5% to 144% in 10-fold diluted postmortem cerebrospinal fluid (pCSF) samples, indicating satisfactory accuracy for quantification despite matrix effects.
Application of the method to pCSF samples from control subjects and AD patients revealed detectable levels of 15 targeted compounds above LOQ values, predominantly downstream metabolites of purine and pyrimidine pathways. Statistical analysis, while showing variability among individual samples, indicated potential differences in nucleotide concentrations between control and AD groups, highlighting the method's utility in biomarker discovery and disease mechanism elucidation.
 MS/MS spectra of representative molecular species using ESI positive mode. (a) Nucleobases, (b) nucleosides and (c) nucleotides
MS/MS spectra of representative molecular species using ESI positive mode. (a) Nucleobases, (b) nucleosides and (c) nucleotides
Typical multiple reaction monitoring (MRM) chromatograms of analytes in postmortem cerebrospinal fluid samples. (a) Control and (b) Alzheimer's disease patient
Reference
- Muguruma, Yoshio, et al. "Comprehensive quantification of purine and pyrimidine metabolism in Alzheimer's disease postmortem cerebrospinal fluid by LC–MS/MS with metal‐free column." Biomedical Chromatography 34.2 (2020): e4722.
How are purines metabolized in the body?
Purines are metabolized through a complex pathway involving enzymatic reactions. Dietary purines (from foods like meat, fish, and legumes) or those synthesized within the body are broken down into uric acid through successive enzymatic steps. Key enzymes in this process include adenosine deaminase, xanthine oxidase, and others responsible for converting purines into intermediate compounds like xanthine and hypoxanthine, which are further metabolized into uric acid. Uric acid is eventually excreted through the kidneys.
What are the functions of purines in the human body?
Purines serve critical roles in cellular processes:
- DNA and RNA Synthesis: Purines (adenine and guanine) are essential building blocks of DNA and RNA, playing pivotal roles in genetic expression and protein synthesis.
- Energy Metabolism: ATP (adenosine triphosphate) and GTP (guanosine triphosphate), derived from purine nucleotides, are key energy carriers in cells, facilitating biochemical reactions and cellular processes.
- Signal Transduction: Purines act as signaling molecules in various pathways, influencing cellular responses to external stimuli and regulating physiological functions like muscle contraction and neurotransmission.
What diseases are associated with abnormal purine metabolism?
Abnormalities in purine metabolism can lead to several health conditions:
- Gout: Elevated levels of uric acid due to inefficient metabolism can crystallize and deposit in joints, causing painful inflammation characteristic of gout.
- Lesch-Nyhan Syndrome: A rare genetic disorder resulting from deficiency of the enzyme hypoxanthine-guanine phosphoribosyltransferase (HGPRT), leading to excessive uric acid production, neurological impairments, and behavioral disturbances.
- Familial Hyperuricemia: Inherited conditions causing elevated uric acid levels without the severe symptoms of gout, predisposing individuals to kidney stones and potential kidney damage.
How do purine nucleotides contribute to cellular energy metabolism?
Purine nucleotides, particularly ATP and GTP, serve as primary energy carriers in cellular metabolism:
- ATP: Produced during cellular respiration, ATP provides energy for essential processes such as muscle contraction, active transport across cell membranes, and biosynthesis of molecules like proteins and lipids.
- GTP: Functions similarly to ATP in specific biochemical reactions, including protein synthesis, signal transduction, and modification of proteins through GTP-binding proteins (G-proteins).
How does purine metabolism relate to gout?
Gout is intricately linked to purine metabolism, specifically the accumulation of uric acid:
- Uric Acid Production: High intake of purine-rich foods or impaired purine metabolism leads to elevated uric acid levels in the blood (hyperuricemia).
- Crystallization and Inflammation: Excess uric acid can crystallize in joints, triggering an inflammatory response characterized by intense pain, swelling, and redness—hallmarks of acute gout attacks.
- Risk Factors: Factors such as genetics, diet rich in purines, obesity, and certain medications can exacerbate uric acid buildup, increasing the risk of gout development.







