- Service Details
- Demo
- Case Study
- FAQ
- Publications
What are Bile Acids?
Bile acids are a large family of steroids, which has a carboxyl group in the side chain. They are predominantly found in the bile of mammals and other vertebrates. In the liver, bile acids can conjugate with taurine or glycine and form bile salts. Chenodeoxycholic acid and cholic acid are the most abundant bile acids in human bile and they are the primary bile acids synthesized in the liver. Deoxycholic and lithocholic acids are the major secondary bile acids and they are formed by the action of intestinal bacterial bacteria on primary bile acids.
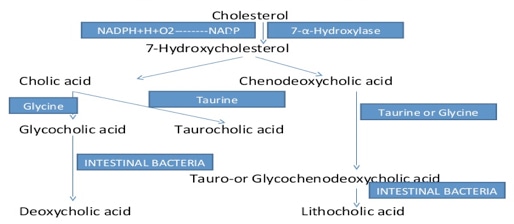
Metabolism of bile acids plays an important role in maintaining and regulating cholesterol homeostasis. About 500 mg of cholesterol is converted to bile salts every day in a healthy adult human. The major pathway for the synthesis of the bile acids in the liver, starts from the conversion of 7alpha-hydroxycholesterol from of cholesterol, while the alternative pathway of bile salt synthesis begins with the formation of 27-hydroxycholesterol or oxysterol-24-hydroxycholesterol. In extra-hepatic tissues, the intermediates generated in these two pathways can be converted to bile salts. These extra-hepatic conversions play an important role in cholesterol homeostasis.
Also, acting as emulsifiers, bile acids facilitate the digestion of triacylglycerol, make lipids more accessible to pancreatic lipases and promote the intestinal absorption of fats (fat-soluble vitamins included).
Various intermediates in bile acids synthesis and the secondary metabolism of these intermediates make bile acids a rather complex mixture. The differences of these compounds in the mixture rely on the number, positions and stereochemistry of hydroxyl groups and the length of the side chain. Not only the concentrations of bile acids in infants, healthy adults and patients with liver disease would be different; the hydroxyl groups may also be introduced at different positions. The complex nature of the bile acids exist in the sample is the key factor determines the analytical method to be used. The bile acids are rather complex mixture and quantification of bile acids is needed. Simple analytical quantification methods have limitations, so time-consuming LC-MS quantification method is needed for bile acids analysis. With LC-MS, variable mixtures of bile acids in blood, urine, meconium, feces and amniotic fluid can be quantified successfully.
Here Creative Proteomics develop a sensitive and rapid method for the analysis of the 66 bile acids physiological samples by LC-MS.
Bile Acids Analysis in Creative Proteomics
Creative Proteomics specializes in comprehensive metabolomics analysis services tailored to meet the rigorous demands of scientific research and clinical investigations. Our expertise covers a wide range of analytical techniques designed to provide precise and reliable quantification of bile acids profiles across various biological samples.
Quantitative Bile Acids Profiling: We employ High-Performance Liquid Chromatography-Mass Spectrometry (HPLC-MS) for accurate measurement and quantification of bile acids, essential for metabolic and disease studies.
Comprehensive Bile Acids Panel: Our service includes thorough analysis of primary and secondary bile acids, aiding in understanding metabolic pathways and disease mechanisms.
Bile Acid Conjugation Analysis: We specialize in the analysis of conjugated bile acids using Gas Chromatography-Mass Spectrometry (GC-MS), crucial for studying bile acid metabolism.
Bile Acid Metabolomics: Utilizing advanced Mass Spectrometry techniques, we provide detailed profiling of bile acid metabolites to support biomarker discovery and clinical research.
Bile Acid Biosynthesis Pathway Analysis: Our capabilities extend to investigating enzymes and intermediates involved in bile acid biosynthesis, supporting genetic disorder research.
Bile Acid Turnover and Dynamics: We measure bile acid turnover rates using isotope labeling techniques combined with HPLC-MS, essential for studying liver function and therapeutic efficacy.

Brochures
Metabolomics Services
We provide unbiased non-targeted metabolomics and precise targeted metabolomics services to unravel the secrets of biological processes.
Our untargeted approach identifies and screens for differential metabolites, which are confirmed by standard methods. Follow-up targeted metabolomics studies validate important findings and support biomarker development.
Download our brochure to learn more about our solutions.
Analytical Techniques for Bile Acids Analysis
High-Performance Liquid Chromatography-Mass Spectrometry (HPLC-MS):
Utilizing the Agilent 1260 Infinity II HPLC System coupled with the Thermo Scientific Q Exactive HF-X Mass Spectrometer. Enables high-resolution separation and sensitive detection of bile acids, facilitating both quantitative analysis and profiling of bile acids species.
Ultra-Performance Liquid Chromatography (UPLC) Coupled with Mass Spectrometry (MS):
Employing the Waters ACQUITY UPLC System coupled with the Waters Xevo G2-XS QTof Mass Spectrometer. Provides enhanced speed, resolution, and sensitivity, ideal for comprehensive bile acids profiling across various sample matrices.
Gas Chromatography-Mass Spectrometry (GC-MS):
Utilizing the Agilent 7890B GC System coupled with the Agilent 5977B GC/MSD System. Well-suited for analyzing bile acids biosynthesis pathways and metabolic intermediates, offering insights into bile acids metabolism.
Nuclear Magnetic Resonance (NMR) Spectroscopy:
Utilizing advanced NMR techniques for structural elucidation and qualitative analysis of bile acids, providing complementary information to chromatographic methods.
List of Bile Acids We Can Analyze Includes, but is not limited to
| Bile Acids Quantified in Our Service | ||
|---|---|---|
| 12-Ketochenodeoxycholic acid | 12-Ketolithocholic acid | 3-Oxocholic acid |
| 6,7-Diketolithocholic acid | 7-Ketodeoxycholic acid | 7-Ketolithocholic acid |
| Allocholic acid | Alloisolithocholic acid | Apocholic acid |
| Chenodeoxycholic acid | Chenodeoxycholic acid 24-β-D-glucuronide | Chenodeoxycholic aicd-3-glucuronide |
| Cholic acid | Cholic acid 3-sulfate | Dehydrocholic acid |
| Dehydrolithocholic acid | Deoxycholic acid | Deoxycholic acid - 3- glucuronide |
| Deoxycholic acid 3-sulfate | DHCA | Dioxolithocholic acid |
| Glycochenodeoxycholic acid | Glycochenodeoxycholic acid 3-sulfate | Glycocholic acid |
| Glycocholic acid 3-sulfate | Glycodehydrocholic acid | Glycodeoxycholic Acid |
| Glycodeoxycholic acid 3-sulfate | Glycohyocholic acid | Glycohyodeoxycholic acid |
| Glycolithocholic acid | Glycolithocholic acid 3-sulfate | Hyodeoxycholic acid |
| Isodeoxycholic acid | Isolithocholic acid | Lithocholic acid |
| Lithocholic acid 3-sulfate | Lithocholic acid-24-glucuronide | Lithocholic acid-3-glucuronide |
| Murocholic acid | Norcholic acid | Nordeoxycholic acid |
| Tauro- β-muricholic acid | Tauro-α-muricholic acid | Tauro-ω-muricholic acid |
| Taurochenodeoxycholic acid | Taurochenodeoxycholic acid 3-sulfate | Taurocholic Acid |
| Taurodehydrocholic acid | Taurodeoxycholic Acid | Taurodeoxycholic acid 3-sulfate |
| Taurohyocholic acid | Taurohyodeoxycholic acid | Taurolithocholic acid |
| Taurolithocholic acid 3-sulfate | THCA | Ursocholic Acid |
| α-Muricholic acid | β-Muricholic acid | λ-Muricholic acid (hyocholic acid) |
| ω-Muricholic acid | ||
Sample Requirements for Bile Acids Analysis
| Sample Type | Recommended Volume | Storage Conditions | Important Considerations |
|---|---|---|---|
| Plasma/Serum | 100 µL | -80°C or liquid nitrogen | Use EDTA or heparin as anticoagulant. Avoid hemolysis. |
| Tissue Samples | 50-100 mg | -80°C or liquid nitrogen | Homogenize in cold PBS. Avoid freeze-thaw cycles. |
| Urine | 500 µL | -80°C or liquid nitrogen | Use clean collection tubes. Avoid contamination with metabolites. |
| Bile Samples | 100-200 µL | -80°C or liquid nitrogen | Collect under sterile conditions to prevent bacterial contamination. |
| Fecal Samples | 50-100 mg | -80°C or liquid nitrogen | Use preservative solutions to maintain bile acids stability. |
Notes:
- Sample Collection: Ensure sterile techniques and appropriate anticoagulants for blood samples.
- Homogenization: Thorough homogenization is critical for tissue and fecal samples to prevent degradation.
- Storage: Maintain samples at -80°C or in liquid nitrogen to preserve bile acids integrity.
- Avoid Contamination: Minimize contamination risks during sample handling to ensure reliable analytical outcomes.
Report
- A detailed technical report will be provided at the end of the whole project, including the experiment procedure, instrument parameters.
- Analytes are reported as uM or ug/mg (tissue), and CV's are generally<10%.
- The name of the analytes, abbreviation, formula, molecular weight and CAS# would also be included in the report.

PCA chart

PLS-DA point cloud diagram

Plot of multiplicative change volcanoes

Metabolite variation box plot

Pearson correlation heat map
Quantifying forms and functions of intestinal bile acid pools in mice.
Journal: International Journal of Molecular Sciences
Published: 2023
Background
The mammalian gastrointestinal tract contains over 100 bile acid species, crucial for lipid absorption. Synthesized in the liver and reabsorbed in the small intestine, bile acids undergo microbial metabolism in the large intestine, influencing health and disease. Dysregulation in bile acid metabolism is implicated in metabolic diseases, GI cancers, inflammatory bowel disease, and cystic fibrosis, highlighting the need for comprehensive analytical approaches to study their roles in physiological and pathological states.
Materials & Methods
Mice
Strains: C57BL/6J (B6) mice from the Jackson Laboratory; B6-derived Slc10a2-/- mice from Dr. Paul Dawson.
Handling: Female mice to avoid fighting; weaned at 4-5 weeks, co-housed at 8-10 weeks, sacrificed at 29-32 weeks.
Compliance: Conducted under IACUC protocols at Scripps Florida or Dartmouth College.
Sample Collection: Mice fasted for 4 hours. Samples (siLC, smvB, feces, pB) were collected, weighed, and stored at -80°C.
Sample Processing:
- Blood: 20 μL mixed with IS solution and 900 μL water, sonicated, and processed through SPE cartridge.
- siLC and Feces: Homogenized, sonicated, centrifuged, and diluted with IS solution.
Quantification: Performed by Creative Proteomics using Agilent 1290 UHPLC and Sciex 4000 QTRAP mass spectrometer. Waters BEH C18 column used for separation.
Data Analysis: Utilized Morpheus for matrix algorithm and Pearson correlation.
Intestinal Explant Cultures
Tissue Preparation: Terminal ileum or proximal colon harvested, cleaned, and cultured on serum-free media-soaked sponges.
Treatment: DMSO, (t)CDCA, GW4064, odevixibat, or endogenous siLC extracts.
Extraction: siLC from B6 or B6.Slc10a2-/- mice homogenized and processed with cholestyramine for BA depletion.
Gene Expression Assays
qPCR: RNA isolated, quantified, and analyzed using SuperScript III Platinum One-Step qRT-PCR Kit and Taqman primer/probe sets.
Nanostring: RNA from spleen, liver, colon, terminal ileum analyzed with custom codeset on nCounter Pro system. Data processed with nSolver software.
Bioinformatics
Metagenomics: Fecal DNA extracted, sequenced using Illumina NovaSeq, and analyzed with One Codex database.
RNA-seq: Total RNA from cultured ileal fragments, sequenced with QuantSeq 3' mRNA-Seq V2 Library Prep Kit, and analyzed with Dartmouth Analytics Core RNAseq pipeline.
Data Analysis: Differential gene expression (DGE) with DESeq2, annotated with org.Mm.eg.db database, visualized with ggplot2.
Mathematical Modeling of Bile Acid Circulation and Metabolism
Model: BA flux through intestine as connected compartments, focusing on reabsorption effects.
Simulation: Using MatLab solver ode113, optimization with fminsearch and sum of squared errors.
Parameters: Active/passive reabsorption rates, bacterial BA metabolism rates optimized to fit experimental data.
Code: Available at github.com/schultz-lab/Bile_Acids.
Results
BA Composition and Depletion Efficiency
Total bile acid (BA) concentrations were measured in intact and BA-depleted extracts from wild type (WT) and Asbt-deficient mice. Intact WT siLC extracts contained approximately 286 μM total BAs, with cholestyramine (CME) treatment efficiently removing about 97% of these BAs. Asbt-deficient extracts had approximately half the total BAs of WT extracts, with CME depleting around 93% of these BAs.
 BA Depletion Efficiency
BA Depletion Efficiency
FXR Activation and Gene Expression
Intact WT siLC extracts induced Fgf15 upregulation similar to tauro-conjugated chenodeoxycholic acid (tCDCA) alone, while this response was attenuated with BA-depleted extracts. RNA-seq analysis revealed fewer than 150 genes significantly affected by BA-replete, but not BA-depleted, siLC extracts, contrasting with over 900 genes influenced by a supraphysiological concentration of tCDCA.
Unique Transcriptional Responses
Approximately 87% of genes regulated by BA-replete siLC extracts showed unique responses to WT or Asbt-deficient BA pools. Asbt-deficient extracts induced more unique transcriptional responses in ileal explants than WT extracts, impacting various physiological processes including prostaglandin metabolism, antimicrobial activity, and xenobiotic metabolism.
 Unique Transcriptional Responses
Unique Transcriptional Responses
Reference
- Sudo, Koichi, et al. "Quantifying forms and functions of intestinal bile acid pools in mice." bioRxiv (2024): 2024-02.
What are the characteristics of bile acids?
Bile acids are crucial for the digestion and absorption of dietary fats and fat-soluble vitamins. They are amphipathic molecules derived from cholesterol in the liver, having both hydrophilic and hydrophobic regions, which enables them to emulsify fats and form micelles.
Structurally, bile acids consist of a steroid core with four fused rings, typically bearing hydroxyl groups at positions 3, 7, and 12. They also possess a side chain ending in a carboxyl group that is conjugated with glycine or taurine to form bile salts in the liver.
The primary bile acids, cholic acid (CA) and chenodeoxycholic acid (CDCA), are synthesized in the liver. In the intestine, these primary bile acids are converted by gut bacteria into secondary bile acids, such as deoxycholic acid (DCA) and lithocholic acid (LCA).
Bile acids play a critical role in fat digestion by emulsifying dietary fats, which allows pancreatic lipase to break down fats into fatty acids and monoglycerides. They form micelles that transport lipids and fat-soluble vitamins (A, D, E, K) to the intestinal mucosa for absorption. Additionally, bile acids help regulate cholesterol levels by promoting its excretion and conversion into bile acids.
The synthesis of bile acids is tightly regulated by feedback mechanisms involving the nuclear receptor FXR (farnesoid X receptor). When bile acid levels are high, FXR activation suppresses further synthesis. Furthermore, bile acids undergo enterohepatic circulation, being efficiently recycled between the intestine and the liver, with approximately 95% reabsorbed in the ileum and returned to the liver.
Clinically, elevated bile acid levels in the blood can indicate liver dysfunction, cholestasis, or bile acid malabsorption. Bile acid derivatives like ursodeoxycholic acid (UDCA) are used to treat certain liver diseases and gallstones.
How should samples be prepared for bile acids analysis?
Sample preparation varies depending on the type of sample (e.g., blood, urine, feces). Typically, it involves steps such as centrifugation, filtration, and sometimes derivatization to improve the detection of bile acids. Proper storage conditions, such as freezing, are essential to preserve sample integrity.
Learn about other Q&A about metabolomics technology.
Quantifying forms and functions of intestinal bile acid pools in mice.
Sudo, K., Delmas-Eliason, A., So ucy, S., Barrack, K. E., Liu, J., Balasubramanian, A., ... & Sundrud, M. S.
Journal: bioRxiv
Year: 2024
Metabolic immaturity of newborns and breast milk bile acids are the central determinants of heightened neonatal vulnerability to norovirus diarrhea.
Peiper, A. M., Morales, J., Phophi, L., Hu, Z., Phillips, M., Williams, C. G., ... & Karst, S. M.
Journal: bioRxiv
Year: 2024
Metabolomic profiling implicates mitochondrial and immune dysfunction in disease syndromes of the critically endangered black rhinoceros (Diceros bicornis)
Corder, M. L., Petricoin, E. F., Li, Y., Cleland, T. P., DeCandia, A. L., Alonso Aguirre, A., & Pukazhenthi, B. S.
Journal: Scientific Reports
Year: 2023
Transcriptomics, metabolomics and lipidomics of chronically injured alveolar epithelial cells reveals similar features of IPF lung epithelium
Willy Roque, Karina Cuevas-Mora, Dominic Sales, Wei Vivian Li, Ivan O. Rosas, Freddy Romero
Journal: bioRxiv
Year: 2020
See more articles published by our clients.







