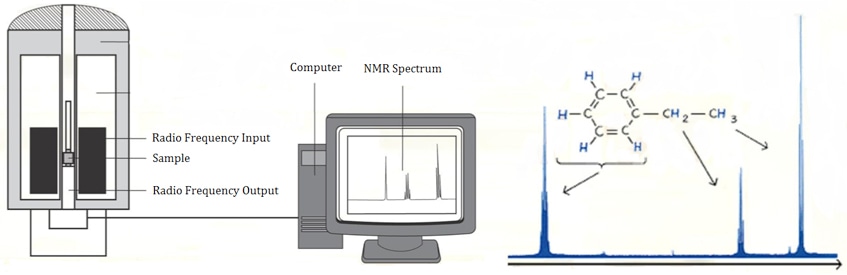
Nuclear Magnetic Resonance (NMR) Spectroscopy has been the dominant analytical technique for structural information of organic compounds and biological macromolecules, because in many cases it can provide valuable structural information of the entire molecules through a set of analytical tests.
Nuclear Magnetic Resonance, is a property of the nucleus of an atom, concerned with nuclear spin (I). Although the isotopes have various I values (including zero), currently the NMR spectroscopy usually works on the nuclei obtaining I = 1/2, including 1H, 13C, 19F and 31P, which facilitates analysis of the most common elements in organic chemistry for structure determination.
When a nucleus with I = 1/2 is placed within an external high frequency magnetic field, it can align itself with the field (lower energy) or against it (higher energy). If pulse radio is applied, nuclei in the lower energy state can absorb the energy and jump to the higher energy state, and then return to lower energy state when the imposed magnetic field disappears. NMR spectroscopy can detect both the absorption, and subsequent release of energy.
With the recent advancement of this technique, NMR spectroscopy can be applied to study
structure information of synthesized molecules
interaction of various molecules
the kinetics or dynamics of molecules
the composition of chemical mixtures
quantitative analysis of specific chemicals
The analytes for NMR spectroscopy range from small organic molecules or metabolite, to mid-sized peptides or a natural product, up to biological macromolecules and synthesized polymers of high molecular weight. As powerful analytical platform, NMR spectroscopy is utilized as alternative to other techniques such as X-ray, crystallography and mass spectrometry for structure information. NMR spectroscopy allows non-destructive, qualitative and quantitative study of molecules both in solution and solid state.







