Title: Chitosan Induces Plant Hormones and Defenses in Tomato Root Exudates
Journal: Front. Plant Sci
Published: https://doi.org/10.3389/fpls.2020.572087
Background
Chitosan, a polymer derived from chitin, is known for its role as a plant defense elicitor. It is commonly used to induce defense mechanisms in plants and is particularly relevant to food security crops like tomato. Chitosan has been shown to promote the accumulation of hormones such as auxin in plant roots. The study focuses on the effect of chitosan on tomato rhizodeposition, specifically the composition of root exudates and their role in plant defense. Through electrophysiological and metabolomic tools, the research explores how chitosan influences plant hormones, lipid signaling, and defense compounds in root exudates, which can help manage soil pathogens like Fusarium oxysporum and Meloidogyne javanica that threaten global crop production. The study highlights the potential of using chitosan for sustainable management of root pests and diseases.
Materials & Methods
Chitosan, Plants, Fungi, and Nematodes
- Chitosan: Used in all experiments with a molecular weight of 70 kDa and 80.5% deacetylation, sourced from Marine BioProducts GmbH (Germany).
- Tomato Plants: Solanum lycopersicum cv. Marglobe.
- Fungi: Pochonia chlamydosporia (isolate number 123) was isolated from Heterodera avenae eggs; Fusarium oxysporum f. sp. radicis-lycopersici strain 4287 was sourced from CBS-KNAW culture collection.
- Nematodes: Meloidogyne javanica obtained from a field population and maintained in tomato plants.
Root Electrophysiology Experiments
Seed Sterilization: Tomato seeds were surface sterilized with 1% sodium hypochlorite and placed in germination medium (GM) with glucose, yeast extract, and agar. Seeds underwent stratification at 4°C for 2 days and germinated at 24°C.
Root Exposure: Plantlets were placed in Gamborg's B5 1:10 medium, and glass microcapillaries filled with 0.5M KCl were inserted into root cells to measure membrane potentials under varying concentrations of chitosan (0.1, 1, and 2 mg/mL).
Root Vital Staining
Staining: Roots were stained with fluorescein diacetate (FDA) and propidium iodide (PI) after exposure to different concentrations of chitosan. Non-damaged cells exhibited green fluorescence (FDA), while damaged cells showed red fluorescence (PI).
Plant Growth Conditions
Experiment 1 (In vitro): Tomato plantlets were grown in sterile cups containing sterilized sand and incubated in a controlled culture chamber. Root exudates were collected after 3 days from plants exposed to chitosan (0.1, 1, 2 mg/mL).
Experiment 2 (In cups): Root exudates were collected at 10, 20, and 30 days after planting (dap). Root systems were washed and exudates collected in sterile plastic containers.
Collection of Root Exudates
Exudates from Experiment 1 were pooled, filtered through Miracloth, and stored at −20°C. For Experiment 2, root exudates were collected by placing plants in SDW and incubating for 24 hours, followed by filtration and freezing.
Emission Excitation Matrix (EEM) Fluorescence Analysis
EEM fluorescence spectra were obtained using a Jasco FP-6500 spectrofluorometer. Data was analyzed using Parallel Factor Analysis (PARAFAC) to identify components related to chitosan treatment.
Root exudates were lyophilized, extracted with methanol, and analyzed for indoleacetic acid (IAA), abscisic acid (ABA), salicylic acid (SA), and jasmonic acid (JA) by UPLC-MS.
Phytomelatonin Detection
Root tissues and exudates were extracted with ethyl acetate, evaporated, and resuspended in acetonitrile. Phytomelatonin was detected using HPLC with fluorescence detection (280/348 nm).
Nuclear Magnetic Resonance (1H NMR)
Root exudates from Experiment 2 were lyophilized and resuspended in deuterated water (D2O) for NMR analysis, performed using a Bruker AVIII 700 MHz spectrometer.
High Performance Liquid Chromatography Electrospray Ionization Tandem Mass Spectrometry (HPLC-ESI-MS)
Tomato root exudates were analyzed by HPLC-ESI-MS, and the data were processed using Partial Least Squares Regression (PLSR) to identify significant m/z variations.
Evaluation of Tomato Root Exudates on Fungi and Nematode Eggs
- Fungal Bioassays: Root exudates were tested against Fusarium oxysporum and Pochonia chlamydosporia conidia in 96-well plates. The OD490 was measured after 4–8 days.
- Nematode Bioassays: Root exudates were tested for their effect on Meloidogyne javanica egg hatching. The hatching percentage was scored after 72 hours.
Statistical Analyses
Data from fluorescence, hormone analyses, and HPLC-ESI-MS were subjected to ANOVA or PCA/PLSR analysis to determine significant differences between treatments. Data were processed using GraphPad Prism and the LIBRA toolbox.
Results
Chitosan Depolarizes Plasma Membrane and Reduces Cell Viability:
- Chitosan at concentrations of 1 and 2 mg/mL depolarizes the plasma membrane of tomato root cells (p < 0.001), as shown by loss of cell viability (PI red staining). At 2 mg/mL, roots exhibit curved morphology and dark precipitates. A lower dose (0.1 mg/mL) does not alter plasma membrane potential significantly but induces some cell damage, with both viable and non-viable cells observed.
- Untreated roots mostly show viable cells with slight red staining due to natural senescence.
Chitosan Induces Hormones and Phenolic Compounds in Root Exudates:
- Fluorescence analysis of root exudates revealed three induced components after chitosan treatment. Component 1 and Component 3, which could be related to salicylic acid (SA) and aromatic amino acids/peptides, were significantly increased (p < 0.01).
- High doses of chitosan (1 and 2 mg/mL) promoted the accumulation of auxin (IAA) and plant defense hormones (SA, jasmonic acid (JA), and abscisic acid (ABA)) in root exudates, with a notable decrease in their accumulation at higher chitosan doses.
- Phytomelatonin levels were slightly elevated by 1 mg/mL chitosan treatment.
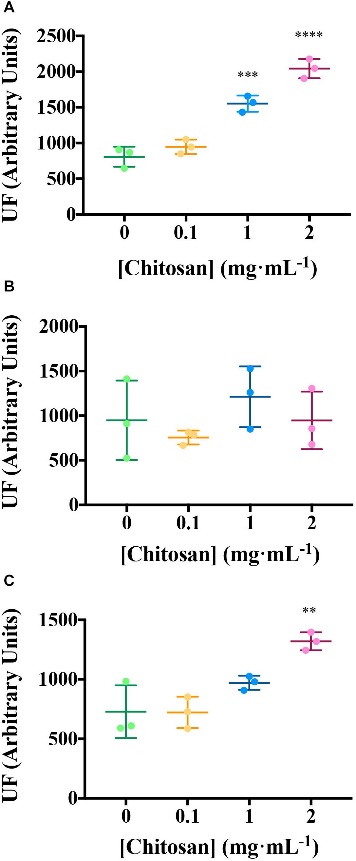 Chitosan increases EEM fluorescence of tomato root exudates.
Chitosan increases EEM fluorescence of tomato root exudates.
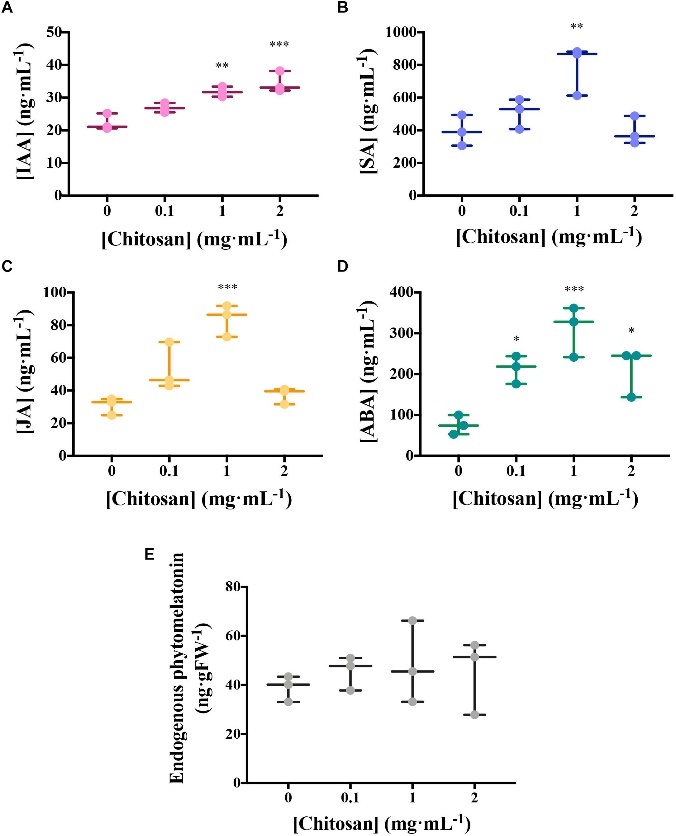 Chitosan induces plant hormones.
Chitosan induces plant hormones.
Chitosan Enhances Exudation of SA and Phenolics:
A low dose of chitosan (0.1 mg/mL) significantly increased the exudation of compounds resembling SA and phenolics after 10 and 20 days of treatment, with a slight reduction in fluorescence intensity at 30 days, possibly due to aging and lignification of roots.
Metabolomic Diversity of Root Exudates:
Metabolomic analysis using NMR showed significant differences in the exudates from treated and untreated plants, especially at 20 days after planting (dap). Specific peaks identified include leucine, acetate, raffinose, glucose, cinnamic acid, and other compounds. Chitosan decreased acetate levels at 20 dap.
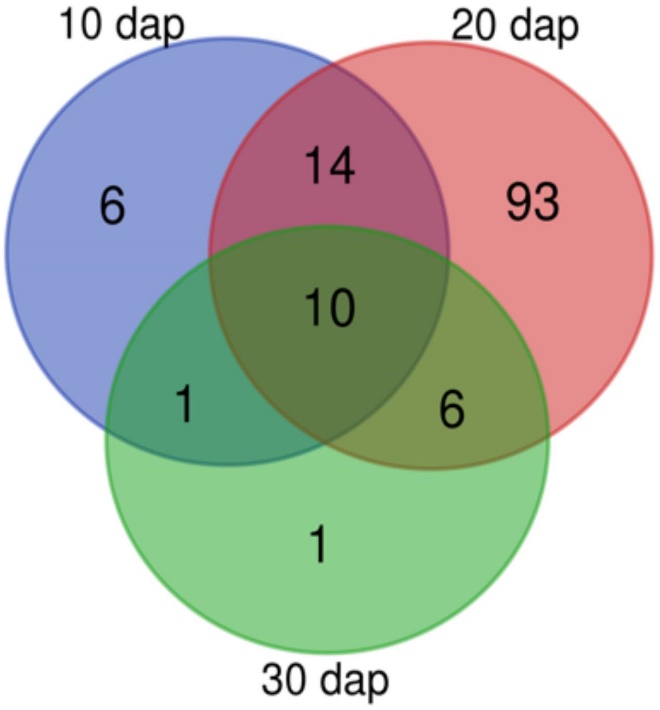 Tomato rhizodeposition varies with time. Venn-diagram of 1H Nuclear Magnetic Resonance analyses peaks detected in tomato root exudates at 10, 20, and 30 days after planting. In all times, plants were treated with 0.1 mg mL– 1 chitosan.
Tomato rhizodeposition varies with time. Venn-diagram of 1H Nuclear Magnetic Resonance analyses peaks detected in tomato root exudates at 10, 20, and 30 days after planting. In all times, plants were treated with 0.1 mg mL– 1 chitosan.
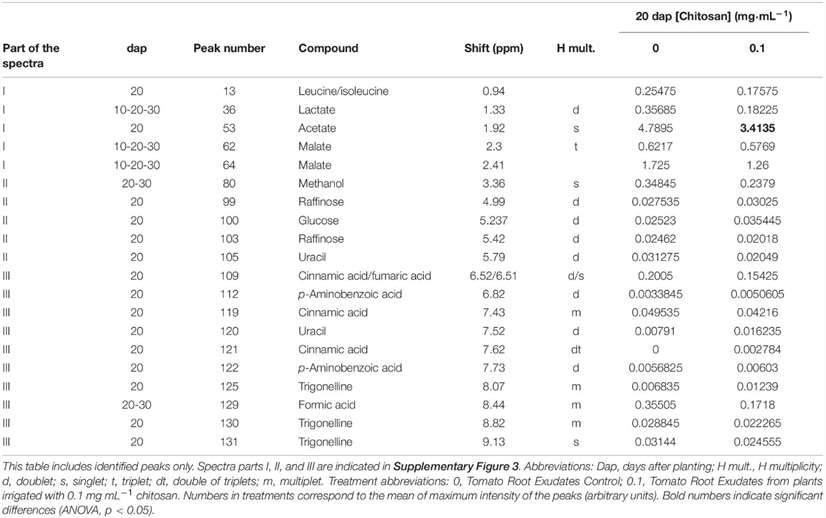 Peak assignments for 1H NMR spectra of tomato root exudates.
Peak assignments for 1H NMR spectra of tomato root exudates.
Lipid Signaling and Defense Compounds:
Lipid signaling compounds, such as oxidized fatty acids, ethyl stearate, and arachidic acid, were elevated by chitosan, while other metabolites like Methyladenosine and N-Fructosyl tyrosine were slightly reduced.
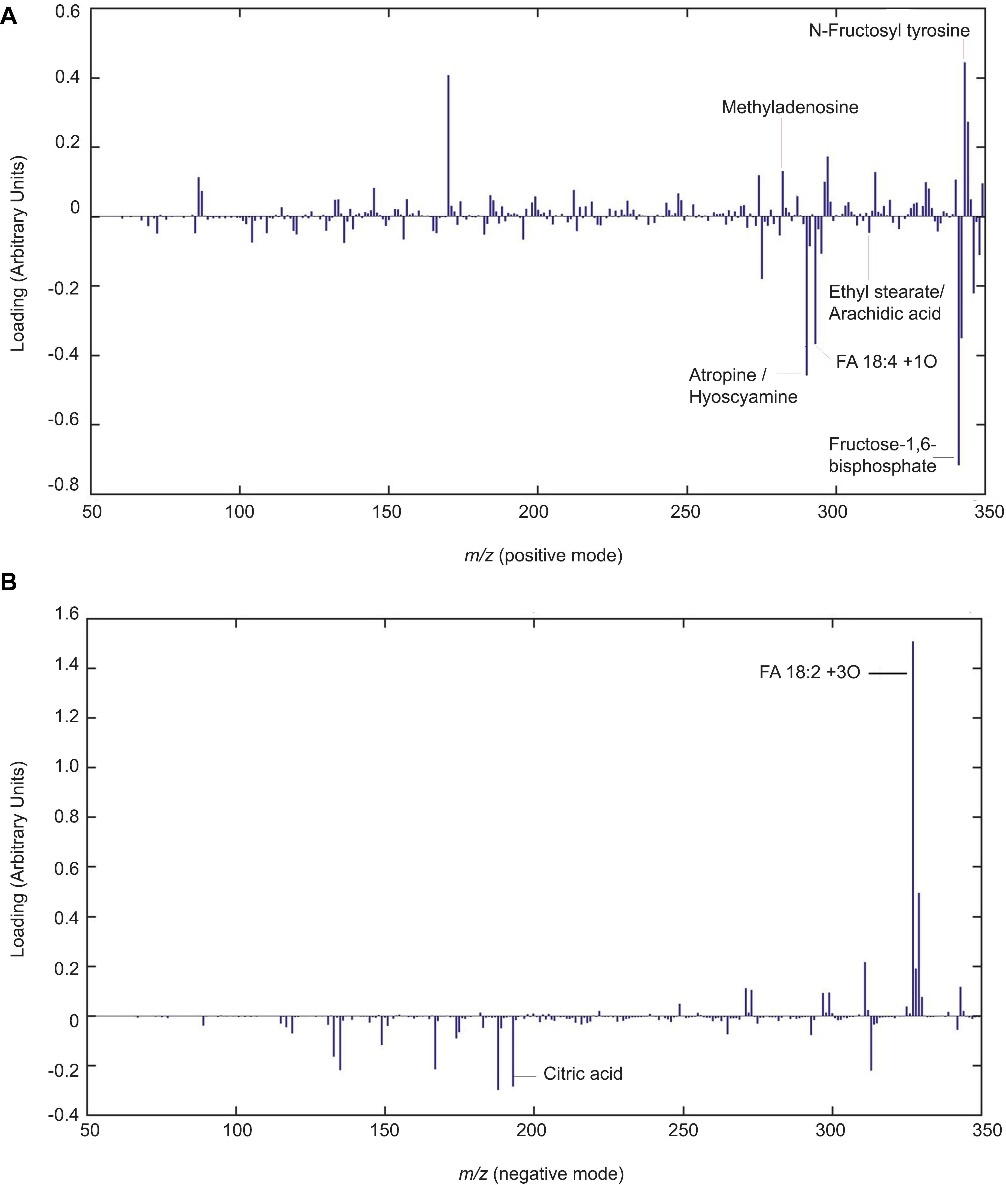 Chitosan induces lipid signaling and defense compounds in tomato root exudates. Partial least squares regression discriminant analysis (PLSLDA) of HPLC-ESI-MS analysis of pools of 20 days after planting tomato root exudates in modes positive (A) and negative (B).
Chitosan induces lipid signaling and defense compounds in tomato root exudates. Partial least squares regression discriminant analysis (PLSLDA) of HPLC-ESI-MS analysis of pools of 20 days after planting tomato root exudates in modes positive (A) and negative (B).
Inhibition of Soil-Borne Pathogens:
Root exudates from chitosan-treated plants inhibited the hatching of root-knot nematode (Meloidogyne javanica) eggs by about 1.5-fold and significantly reduced the growth of Fusarium oxysporum f.sp. radicis-lycopersici (FORL). The exudates had no significant effect on the growth of Pochonia chlamydosporia, a biocontrol fungus.
Reference
- Gedük, Aysun Şener, and Selma Atsız. "LC-MS/MS phenolic composition of peach (Prunus persica (L.) Batsch) extracts and an evaluation of their antidiabetic, antioxidant, and antibacterial activities." South African Journal of Botany 147 (2022): 636-645. https://doi.org/10.1016/j.sajb.2022.02.026






