- Service Details
- Demo
- Case Study
- FAQ
- Publications
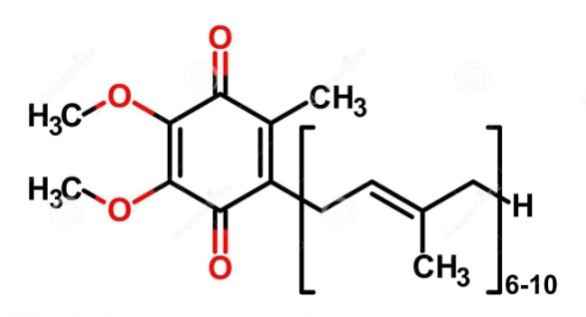
What Is CoQ10
Coenzyme Q10, also known as ubiquinone, ubidecarenone, coenzyme Q, and is abbreviated to CoQ10. It is a 1,4-benzoquinone, where Q refers to the quinone chemical group and 10 refers to the number of isoprenyl chemical subunits in its tail. CoQ10 is a coenzyme which is ubiquitous in the bodies of most animals, especially on the membranes of many organelles.
Coenzyme Q10 (CoQ10) exists in two isomeric forms: Reduced Coenzyme Q10 (CoQ10H2) and Oxidized Coenzyme Q10 (CoQ10). These isomers exhibit mirror-image three-dimensional structures. CoQ10H2 functions as a potent free radical scavenger, effectively inhibiting lipid and protein peroxidation reactions, ultimately resulting in the formation of Oxidized Coenzyme Q10 (CoQ10).
Oxidized Coenzyme Q10 (CoQ10) is a lipid-soluble compound with widespread presence in biological organisms. It primarily resides on the inner mitochondrial membrane, where it actively participates in crucial processes like electron transport within the respiratory chain, antioxidation, and metabolic regulation. It serves as a natural antioxidant and proficiently eliminates free radicals. In clinical practice, Coenzyme Q10 is frequently employed for adjunctive therapy in various conditions, including hypertension, chronic heart failure, ischemic cardiomyopathy, hepatitis, and cancer.
Conversely, Reduced Coenzyme Q10 (CoQ10H2) acts as an activator of cellular metabolism and functions as an immunostimulant. It possesses remarkable antioxidant properties and demonstrates the capacity to eliminate free radicals. In clinical settings, CoQ10H2 finds extensive application in the treatment of cardiovascular diseases, neurological disorders, hypertension, hyperlipidemia, diabetes, severe hepatitis, and various other medical conditions.
CoQ10 is well defined as a crucial component of the electron transport chain and participates in aerobic cellular respiration in mitochondria which converts biochemical energy from nutrients into adenosine triphosphate (ATP) and about ninety-five percent of the human body's energy is generated this way. CoQ10 functions as an electron carrier from enzyme complex I and enzyme complex II to complex III in aerobic cellular respiration. This is very important in the process, since no other molecule can perform this function. Thus, the primary function of CoQ10 in every cell of the body is in generating energy. CoQ10 continuously goes through an oxidation–reduction cycle as an energy carrier and it becomes reduced when it accepts electrons, as it gives up electrons, it becomes oxidized. CoQ10 can act as an antioxidant because the CoQ10 molecule can give up one or both electrons quite easily in its reduced form.
Coenzyme Q10 Analysis Services Offered by Creative Proteomics
Quantitative CoQ10 Measurement
We offer precise quantification of CoQ10 levels in various biological samples, including blood, plasma, tissue, and cell cultures. Our state-of-the-art equipment ensures highly accurate and reproducible results, essential for clinical research and product development.
Oxidized and Reduced CoQ10 Form Analysis
CoQ10 exists in two forms: the oxidized form (ubiquinone) and the reduced form (ubiquinol). Both forms are biologically active and play distinct roles in cellular function. We provide separate quantification of these forms to give a comprehensive understanding of their role in different physiological contexts.
Pharmacokinetics and Bioavailability Studies
For companies involved in the development of CoQ10 supplements or therapeutics, we offer pharmacokinetic profiling and bioavailability studies. These services help in determining the absorption, distribution, metabolism, and excretion (ADME) of CoQ10 formulations.
Custom CoQ10 Assays
We understand that different research projects may have unique requirements. That's why we provide custom assay development services to tailor CoQ10 analysis protocols to your specific needs.
Nutritional and Cosmetic Formulation Analysis
For companies in the nutrition and cosmetics industries, we provide analysis of CoQ10 levels in various products, including dietary supplements and skin care formulations, ensuring compliance with regulatory standards and labeling accuracy.
Analytical Techniques for Coenzyme Q10 Analysis
At Creative Proteomics, we use advanced analytical methods to ensure accurate and reliable CoQ10 analysis:
High-Performance Liquid Chromatography (HPLC)
HPLC, paired with photodiode array (PDA) detection or mass spectrometry (MS), provides high sensitivity and specificity for measuring CoQ10 and its related compounds in biological and nutritional samples.
Liquid Chromatography-Mass Spectrometry (LC-MS/MS)
LC-MS/MS offers superior sensitivity and allows simultaneous detection of oxidized and reduced CoQ10 forms. This technique is especially valuable for analyzing CoQ10 in complex biological matrices.
Gas Chromatography-Mass Spectrometry (GC-MS)
GC-MS is used for detecting CoQ10 derivatives and precursors, offering detailed metabolic profiling and analysis of volatile or derivatized CoQ10 compounds.
UV-Vis Spectrophotometry
UV-Vis spectrophotometry provides quick quantification of CoQ10 in routine quality control, leveraging CoQ10's strong absorbance in the UV range.
Sample Requirements
Animal and Clinical Tissue Specimens: 200 mg/sample, flash-frozen in liquid nitrogen, stored at -80°C, and shipped with dry ice to avoid repeated freeze-thaw cycles.
Serum and Plasma: 200 μL/sample, flash-frozen in liquid nitrogen, stored at -80°C, and shipped with dry ice.
Urine: 1 mL/sample, flash-frozen in liquid nitrogen, stored at -80°C, and shipped with dry ice.
Microbes and Cells: 1×107 cells/sample, flash-frozen in liquid nitrogen, stored at -80°C, and shipped with dry ice.
Fecal Samples: 200 mg/sample, flash-frozen in liquid nitrogen, stored at -80°C, and shipped with dry ice.
For other sample types, please consult our technical support or sales team.
Note: Avoid repeated freeze-thaw cycles.

PCA chart

PLS-DA point cloud diagram

Plot of multiplicative change volcanoes

Metabolite variation box plot

Pearson correlation heat map
Simultaneous analysis of retinol, six carotenoids, two tocopherols, and coenzyme Q10 from human plasma by HPLC
Journal: Journal of Chromatography B
Published: 2020
Background
Lipophilic antioxidants such as vitamins A and E, carotenoids, and coenzyme Q10 play essential roles in human health due to their antioxidant properties. Accurately measuring these compounds in plasma is important for assessing nutritional status and oxidative stress. High-performance liquid chromatography (HPLC) is a well-established method for quantifying these antioxidants, but resolving carotenoid isomers and achieving high sensitivity for all compounds can be challenging. This study aimed to develop a robust HPLC method with UV detection to simultaneously quantify these antioxidants in human plasma samples.
Materials & Methods
The method combines liquid-liquid extraction with HPLC-UV for the simultaneous analysis of lipophilic antioxidants, including vitamins A and E, carotenoids, and coenzyme Q10.
Sample Preparation: Plasma samples were deproteinized using ethanol, followed by extraction using hexane. A 9‰ NaCl solution was added to enhance the separation between the aqueous and organic phases. Butylated hydroxytoluene (BHT) was used as a stabilizer to prevent oxidation during the process. The lipid-soluble compounds were extracted from the organic phase.
Chromatographic Conditions: A C30 column was used to achieve high resolution between carotenoid isomers, which are often difficult to separate. The column provided improved separation for closely related compounds, such as lutein and zeaxanthin. UV detection was set at four different wavelengths to detect different classes of antioxidants.
Internal Standards: Three internal standards were chosen to represent each class of compounds: lipophilic vitamins, carotenoids, and coenzyme Q10. These standards were chemically similar to the target compounds, allowing for accurate and reproducible quantification.
Metabolomics Relevance: This method is particularly relevant to metabolomics research, where precise quantification of bioactive compounds like lipophilic vitamins and carotenoids is crucial for understanding metabolic profiles, oxidative stress, and their relationship to health and disease. The HPLC method facilitates metabolite profiling in clinical and nutritional studies by providing accurate measurements of lipid-soluble antioxidants.
Results
A high resolution was achieved between various carotenoids, especially lutein and zeaxanthin, which are often difficult to separate. The method also successfully separated α- and γ-tocopherol, though β- and γ-tocopherols were not fully resolved.
The recovery rates for all compounds were within the acceptable range of 90% to 110%, with values ranging from 93.1% for coenzyme Q10 to 108.0% for lycopene.
Intra-assay precision was better than 5% for most compounds, except for γ-tocopherol and zeaxanthin, while inter-assay precision was within 15% for all compounds, which meets the regulatory requirements for biological variation.
Limits of quantification (LOQ) for retinol, α-tocopherol, γ-tocopherol, coenzyme Q10, and carotenoids were found to be similar to previously reported values in the literature.
Mean plasma concentrations of antioxidants in a French adult cohort (n=2307) were reported as 2.1 µmol/L for retinol, 27.2 µmol/L for α-tocopherol, and varying concentrations for carotenoids, closely matching values reported in other population studies.
 Figure 1: Representative HPLC chromatograms for (A) extracted standards of retinol, gamma tocopherol, alpha-tocopherol, carotenoids and coenzyme Q10 with their internal standards (Q4, alpha-tocopherol nicotinate, and echinenone), and for (B) extracted human plasma at the four wavelengths of detection.
Figure 1: Representative HPLC chromatograms for (A) extracted standards of retinol, gamma tocopherol, alpha-tocopherol, carotenoids and coenzyme Q10 with their internal standards (Q4, alpha-tocopherol nicotinate, and echinenone), and for (B) extracted human plasma at the four wavelengths of detection.
 Figure 2: HPLC chromatograms at 292 nm for (A) gamma and alpha tocopherols, (B) ß-tocopherol alone, and (C) a mixture of gamma, alpha and ß-tocopherols.
Figure 2: HPLC chromatograms at 292 nm for (A) gamma and alpha tocopherols, (B) ß-tocopherol alone, and (C) a mixture of gamma, alpha and ß-tocopherols.
Reference
- Boulet, Lysiane, et al. "Simultaneous analysis of retinol, six carotenoids, two tocopherols, and coenzyme Q10 from human plasma by HPLC." Journal of Chromatography B 1151 (2020): 122158.
Why is HPLC preferred for CoQ10 analysis, and are there other methods?
High-performance liquid chromatography (HPLC) is preferred for CoQ10 analysis due to its high precision, sensitivity, and ability to distinguish between oxidized and reduced forms of CoQ10. HPLC offers accurate quantification even in complex biological matrices such as plasma, tissue, and cells. Alternative methods like mass spectrometry (MS) or liquid chromatography-tandem mass spectrometry (LC-MS/MS) can also be used, especially when higher specificity or multiplexing with other metabolites is required. However, HPLC remains the gold standard due to its robustness and reliability in routine CoQ10 quantification.
Can CoQ10 analysis provide insight into aging and age-related diseases?
Yes, CoQ10 levels naturally decline with age, and this reduction is linked to decreased mitochondrial efficiency, increased oxidative stress, and the onset of age-related diseases such as cardiovascular disorders, neurodegeneration, and metabolic syndromes. Monitoring CoQ10 levels in aging populations can help identify individuals at risk for these conditions and provide guidance on supplementation to slow down or mitigate the effects of aging on cellular energy production and antioxidant defense mechanisms.
What factors should be considered when preparing samples for CoQ10 analysis?
Proper sample preparation is crucial for accurate CoQ10 analysis. Key factors include minimizing exposure to light and oxygen to prevent oxidation of the reduced CoQ10H2 form, using flash freezing methods (e.g., liquid nitrogen) to preserve sample integrity, and storing samples at -80°C. Additionally, repeated freeze-thaw cycles should be avoided to prevent degradation. For plasma samples, fasting is recommended to avoid dietary interference with CoQ10 levels. Ensuring optimal sample handling conditions is vital for obtaining reliable results.
Can CoQ10 analysis be performed on non-human samples, such as plants or microorganisms?
Yes, CoQ10 analysis can be performed on a wide range of non-human biological samples, including plant tissues, yeast, and other microorganisms. CoQ10 is present in most aerobic organisms as part of their mitochondrial electron transport chain. Analyzing CoQ10 in these systems can provide insights into energy metabolism, oxidative stress response, and bioenergetic health across different species. This capability is particularly useful in agricultural research, industrial fermentation processes, and comparative bioenergetics studies.
What is the typical turnaround time for CoQ10 analysis, and how are the results delivered?
The typical turnaround time for CoQ10 analysis at Creative Proteomics is 1 to 2 weeks, depending on the complexity of the sample type and the analysis required. Once the analysis is completed, clients receive a detailed technical report, which includes quantification of both oxidized and reduced CoQ10 forms, an interpretation of the results, and any recommendations for further research or action. Data is provided in both raw and processed formats, allowing clients to integrate the findings into broader studies or clinical decisions.
Comparative metabolite profiling of salt sensitive Oryza sativa and the halophytic wild rice Oryza coarctata under salt stress.
Tamanna, Nishat, et al.
Journal: Plant‐Environment Interactions
Year: 2024
https://doi.org/10.1002/pei3.10155
The Brain Metabolome Is Modified by Obesity in a Sex-Dependent Manner.
Norman, Jennifer E., et al.
Journal: International Journal of Molecular Sciences
Year: 2024
https://doi.org/10.3390/ijms25063475
Regulation of host metabolism and defense strategies to survive neonatal infection.
Wu, Ziyuan, et al.
Journal: bioRxiv
Year: 2024
https://doi.org/10.1016/j.bbadis.2024.167482








