Glycosylation refers to the process by which proteins or lipids are linked to glycan chains by the action of enzymes. As an important post-translational modification, glycosylation is involved in and regulates many life activities of living organisms. With the continuous development of proteomic technology, studies for protein glycosylation have received extensive attention. Glycobiology is the study of glycan formations, structures, and functions and has become an area of interest for life sciences that cannot be ignored at present.
Principle
Hydrophilic interaction liquid chromatography (HILIC) is a glycopeptide enrichment technology based on the strong hydrophilic properties of polyhydroxy-rich glycan chains, meanwhile utilizing chromatography to separate glycopeptides from non-glycosylated peptides. HILIC remains unbias for different types of glycopeptides and can maintain the integrity of the sugar chain structure, which is suitable for studying the complete glycopeptide structures.
The identification of a single N-glycoprotein: After protein cleavage into peptides by trypsin, glycopeptides are enriched using the HILIC assay. N-glycosaminidase (PNGase) cleaves between the innermost GlcNAc and asparagine residues of high mannose, hybrid, and complex oligosaccharides. Then, PNGase F was removed, and mass spectrometry was performed. The identification of glycosylated proteins and modification sites in complex samples is performed using the Byonic method. The Byonic search engine provides a sensitive and comprehensive identification of peptides and proteins, which is accompanied by the PreviewTM software allows rapid assessment of peptide quality errors, digestion specificity, and modifications.
Our Glycoproteomics (Glycopeptides Analysis) Services
Creative Proteomics combines reductase digestion, N-glycosylated peptide enrichment, deglycosylation in H2O18, and highly sensitive LC-MS detection techniques to analyze and clarify N-glycosylation modification sites of samples. The deglycosylated N-glycan chains can also be interpreted by the MALDI-TOF, which can provide relevant experimental evidence, including XICs mass spectra, MS2 spectra, and MALDI-TOF glycan spectra to support data analysis.
Workflow of Glycoproteomics
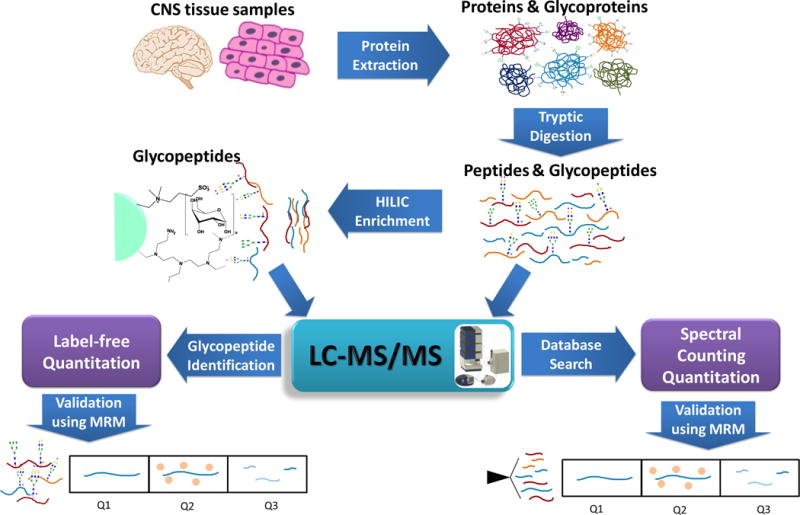
LC-MS/MS approaches of Proteomics and Glycoproteomics (Rui Zhu et al. 2018)
Service Contents
Analysis of N-Glycan modification sites (optional O18 labeling)
MALDI-TOF Glycopeptide Mapping (including N-Glycan and O-Glycan)
Glycoprotein Profiling of Intact Proteins
Glycoform Ratio Analysis
Analysis of glycosylation sites and glycoforms
Advantages
- Professional technical support
- Experienced operators
- Independently developed and advanced experimental SOPs
- Rich experience in animal & plant analysis
- Strong bioinformatics team
Applications
- Identification of post-translational modifications of proteins from animal tissues, cells, organelles, blood, plasma, urine, cerebrospinal fluid, saliva, and other body fluids
- Post-translational modification identification of extracted proteins from plants (rice, Arabidopsis, wheat, etc.)
- Identification of protein post-translational modifications from yeast, E. coli
- Other common biological protein samples
Platforms
MALDI-TOF, Q-Exactive Plus, Q-Exactive HF, Orbitrap Fusion, Orbitrap Fusion Lumos
Sample requirements
- Tissue samples: animal, microbial tissue wet weight > 30 mg; plant fresh tissue > 300 mg
- Cell samples: cell volume > 107
- Body fluid samples: serum volume > 1500 μL
- Protein extracts: total protein > 200 μg and concentration > 1 μg/μL
- SDS-PAGE gel samples: total target protein > 50 μg
Delivery
- Experimental report
- Raw data
- Experimental results






